The global spread of the COVID-19 pandemic and the resulting economic impact and public health burden has highlighted the urgent need for a vaccine to prevent the disease. The challenges associated with rapidly developing and producing a vaccine at a large scale are, however, enormous.
Verdict has conducted a poll to assess the time that could be taken for the approval of a COVID-19 vaccine and its availability to the public.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
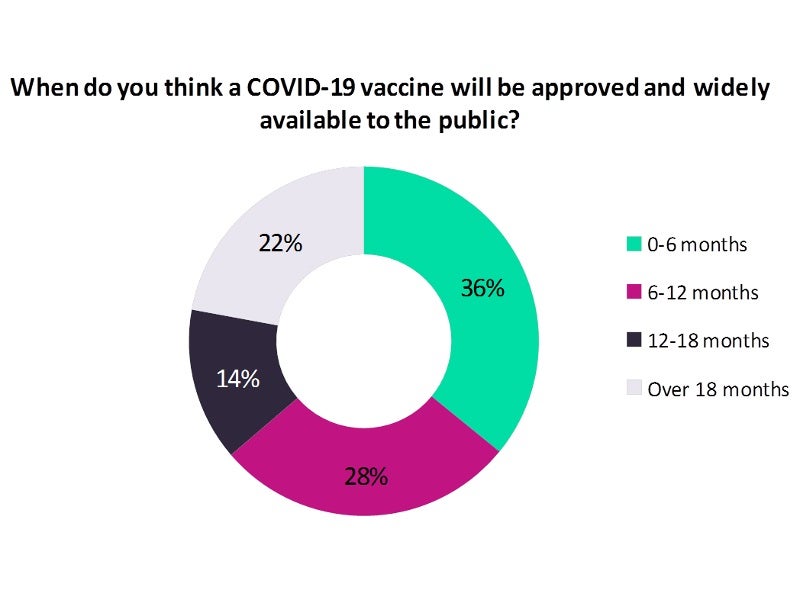
Analysis of the results shows a high chance for an approved COVID-19 vaccine to be available over the next six months, as opined by a majority 36% of the poll respondents, whereas 28% of the respondents feel it could take six to 12 months for the same.
Less than one-fourth (22%) of the poll respondents opined that the approval and availability of a COVID-19 vaccine could take between 12 and 18 months, while 14% felt that it could take more than 18 months.
The analysis is based on 875 responses received from readers of Verdict’s Pharmaceutical Technology site between 18 June and 01 July.


US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe poll findings are in line with those of another poll conducted by Verdict earlier in April, which found high confidence about the possible development of a COVID-19 vaccine within 12 months.
COVID-19 vaccine approval and availability
Research organisations and pharmaceutical companies have joined hands in developing a vaccine against the COVID-19 virus that has affected millions of people.
Existing drugs such as remdesivir have been granted emergency use authorisation (EUA) to treat the disease, meanwhile.
Antimalarial drugs including chloroquine and hydroxychloroquine were also awarded EUA, but later revoked after studies showed that they were ineffective in treating the disease.
High-risk groups should be given priority access to vaccine, upon licensure: Poll
In another poll conducted on Pharmaceutical Technology site, Verdict tried to assess readers’ opinion about who should be given priority access to a COVID-19 vaccine upon licensure.
The poll options included three groups namely those with high-risk such as the elderly and patients with co-morbidities, everyone, and those in high-risk geographies from infection.
A majority 46% of the respondents opined that elderly and people with co-morbidities should be given priority access, followed by 33% who opined that everyone should have the same access to the vaccine.
Prioritising the vaccine’s accessibility to high-risk geographies is of high importance for just 21% of the respondents.

The analysis is based on 354 responses received between 18 June and 08 July.
COVID-19 vaccines in advanced clinical trials
Eight vaccines are in phase three/two clinical trials currently, while another 120 are in pre-clinical evaluation, according to the World Health Organization (WHO).
The vaccines that have reached advanced clinical trials include ChAdOx1-S by University of Oxford and AstraZeneca, an adenovirus type 5 vector vaccine by CanSino Biological and Beijing Institute of Biotechnology, mRNA-1273 vaccine by Moderna, and National Institute of Allergy and Infectious Diseases.
Sinopharm is collaborating with the Wuhan Institute of Biological Products and Beijing Institute of Biological Products to develop an inactivated vaccine.
Sinovac is developing an inactivated vaccine with alum and Novavax is developing a full-length recombinant SARS CoV-2 glycoprotein nanoparticle vaccine, while BioNTech, Fosun Pharma, and Pfizer are developing a vaccine based on 3 LNP-mRNAs.





