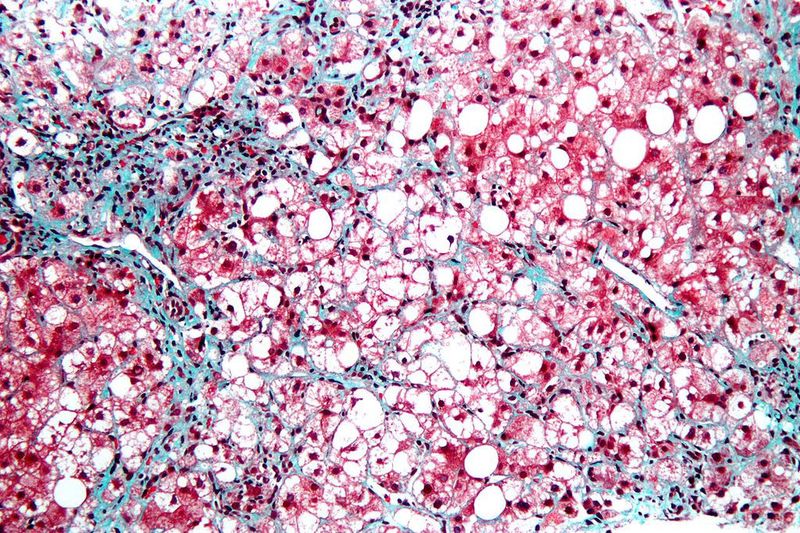
CymaBay Therapeutics has reported top-line, 12-week results from an ongoing Phase IIb clinical trial of seladelpar to treat nonalcoholic steatohepatitis (NASH).
Seladelpar has been designed as a selective agonist of peroxisome proliferator-activated receptor delta (PPARδ) to treat NASH and primary biliary cholangitis (PBC).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Data showed that patients treated with the drug candidate experienced minimal and non-significant reductions in liver fat compared to placebo.
Meanwhile, reductions in liver injury markers were observed to be robust and clinically meaningful.
The double-blind, placebo-controlled, 52-week trial enrolled 181 patients with biopsy-confirmed NASH who received 10mg, 20mg or 50mg once-daily seladelpar or placebo.
The primary endpoint of the study was the relative change in liver fat content from baseline to 12 weeks, measured by magnetic resonance imaging-proton density fat fraction (MRI-PDFF).

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe safety and tolerability profile of seladelpar appeared to be favourable across all doses. The most common treatment-emergent adverse events were nausea, constipation, dizziness, headache, gastroesophageal reflux disease, and upper abdominal pain.
CymaBay Therapeutics chief medical officer Dr Pol Boudes said: “While the reductions in liver fat were minimal, we remain encouraged by the significant improvements in biochemical markers of liver injury that we observed at week 12.
“The observed improvement in markers of liver injury are consistent with the observed effects of seladelpar in PBC and further support the potential for seladelpar to improve liver health.”
The company will continue the trial to 52 weeks and the evaluations will involve a liver biopsy, non-invasive imaging evaluations and biomarker assessments of inflammation and fibrosis.
Secondary efficacy measures of the study include the assessment of histological improvement in NASH and fibrosis by comparing liver biopsy samples obtained at baseline and 52 weeks.





