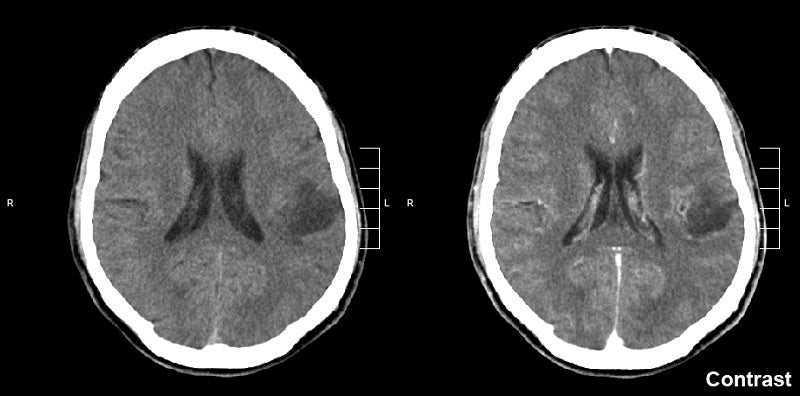
Day One Biopharmaceuticals has reported positive topline data from the pivotal Phase II FIREFLY-1 trial of investigational agent tovorafenib (DAY101) in recurrent or progressive paediatric low-grade glioma (pLGG).
The open-label pivotal trial has been designed for assessing tovorafenib as a once-weekly monotherapy in recurrent or progressive pLGG patients aged six months to 25 years.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It is intended to support tovorafenib’s potential regulatory approval and is being conducted in partnership with the Pacific Pediatric Neuro-Oncology Consortium (PNOC).
Findings from the trial showed an overall response rate (ORR) of 64%, and a clinical benefit rate (CBR) of 91% was observed in 69 heavily pretreated, RANO-evaluable patients.
The company stated that 86% of patients had a BRAF fusion alteration while the remaining 14% had a BRAF mutation.
Among 77 treated patients, the median duration of tovorafenib treatment was 8.4 months, with 77% of patients on treatment.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIn the trial, approximately 60% of patients had received at least one MAPK inhibitor before participating.
Day One co-founder and chief medical officer Samuel Blackman said: “The responses we’ve observed in the FIREFLY-1 study with weekly monotherapy tovorafenib in children with recurrent or progressive low-grade gliomas are very encouraging.
“As tovorafenib progresses in the clinic, we want to thank the patients, their families, the clinical investigators, and the advocates who have chosen to participate in the FIREFLY-1 clinical trial and support the development of a potential new treatment for children in need of new therapeutic options.”
In the first half of this year, the company intends to submit a New Drug Application for tovorafenib based on tovorafenib’s efficacy and safety profile observed in the FIREFLY-1 trial.
It is also expanding to develop tovorafenib as a front-line therapy to treat recently diagnosed pLGG patients.





