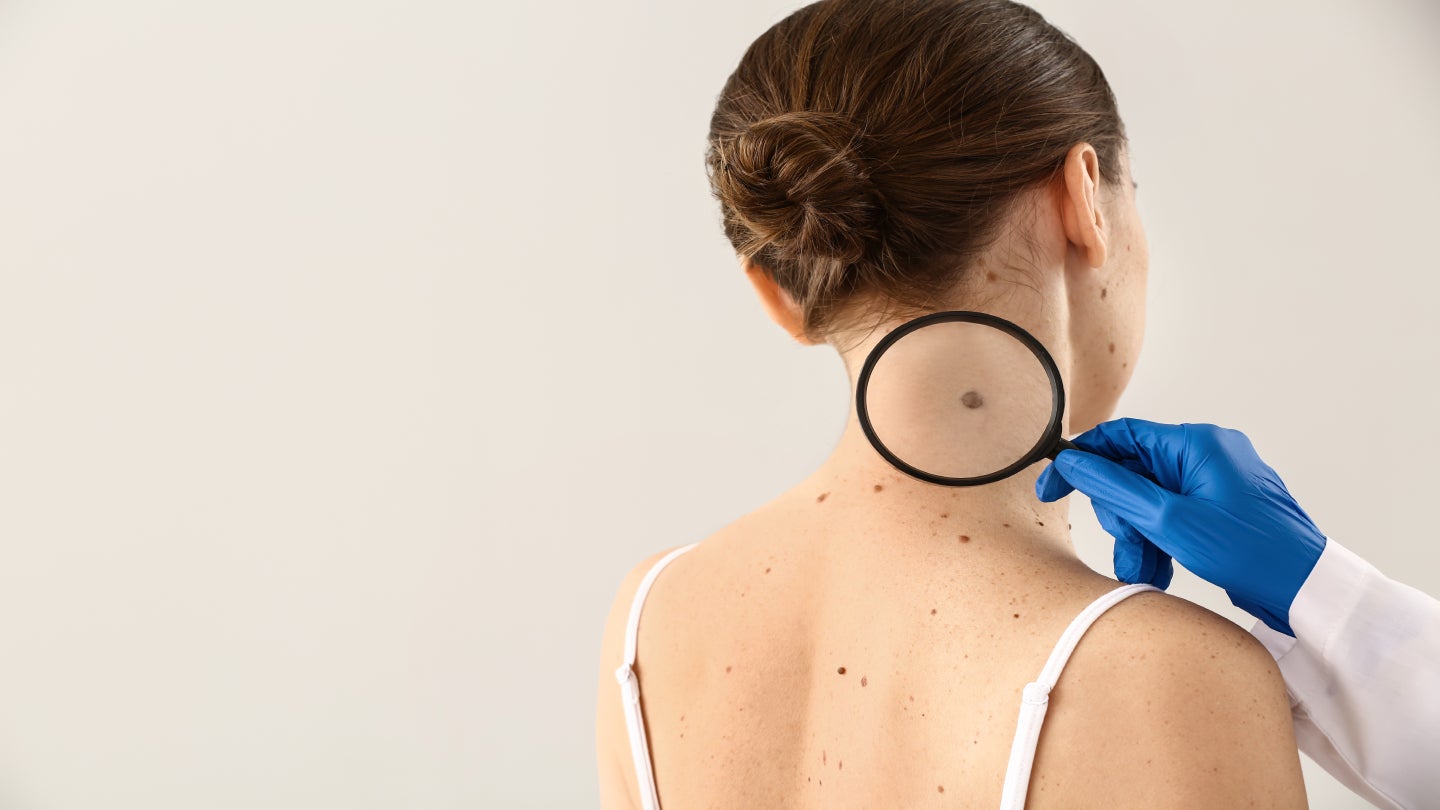
The California-based DermTech has announced positive results from its Trust 2 study, evaluating its system for ruling out melanoma showing a negative predictive value (NPV) of 99.7%.
DermTech reported positive topline results from the Trust 2 trial, which examined the gene expression assay component of the DermTech Melanoma Test (DMT).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The study results demonstrated an NPV of 99.7% for the foundational gene expression assay component of the company’s melanoma test. The study results also included a sensitivity of 95.8%, a specificity of 69.4% and a positive predictive value (PPV) of 13.4%.
Launched in 2021, the Trust 2 study is a follow-up to the company’s previous real-world study Trust 1. The first trial was published in 2021, examining the evaluated tested lesions of more than 1,500 patients.
The Trust 2 study enrolled more than 20,000 patients previously tested with DMT in a real-world clinical setting. The follow-up evaluations occurred for 5,000 tested lesions with median and mean follow-up durations of 348 days and 337 days, respectively.
These follow-up examinations included pathology diagnoses for lesions that were previously biopsied. Also, it included re-examination for lesions that were monitored rather than biopsied, in which the lesion was classified as either stable or changed in a manner determined as concerning for melanoma.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataLoren Clarke, chief medical officer for DermTech, said: “A high NPV delivers assurance that a suspicious pigmented lesion which tests negative is unlikely to be a melanoma. As a non-invasive test that has demonstrated an NPV of 99% or higher in multiple, large studies, the DMT provides actionable genomic information for a suspicious pigmented lesion that a clinician may be hesitant to biopsy for a variety of reasons.”
GlobalData’s Medical Device Intelligence Centre details how the market for cancer screening tests is set to hit $1.8bn by the end of this year, with that figure expected to rise to $2.17bn by the end of 2030.
GlobalData is the parent company of Clinical Trials Arena.
In November 2023, competitor DermaSensor announced results from a study examining its own artificial intelligence-powered system for detecting skin cancers.





