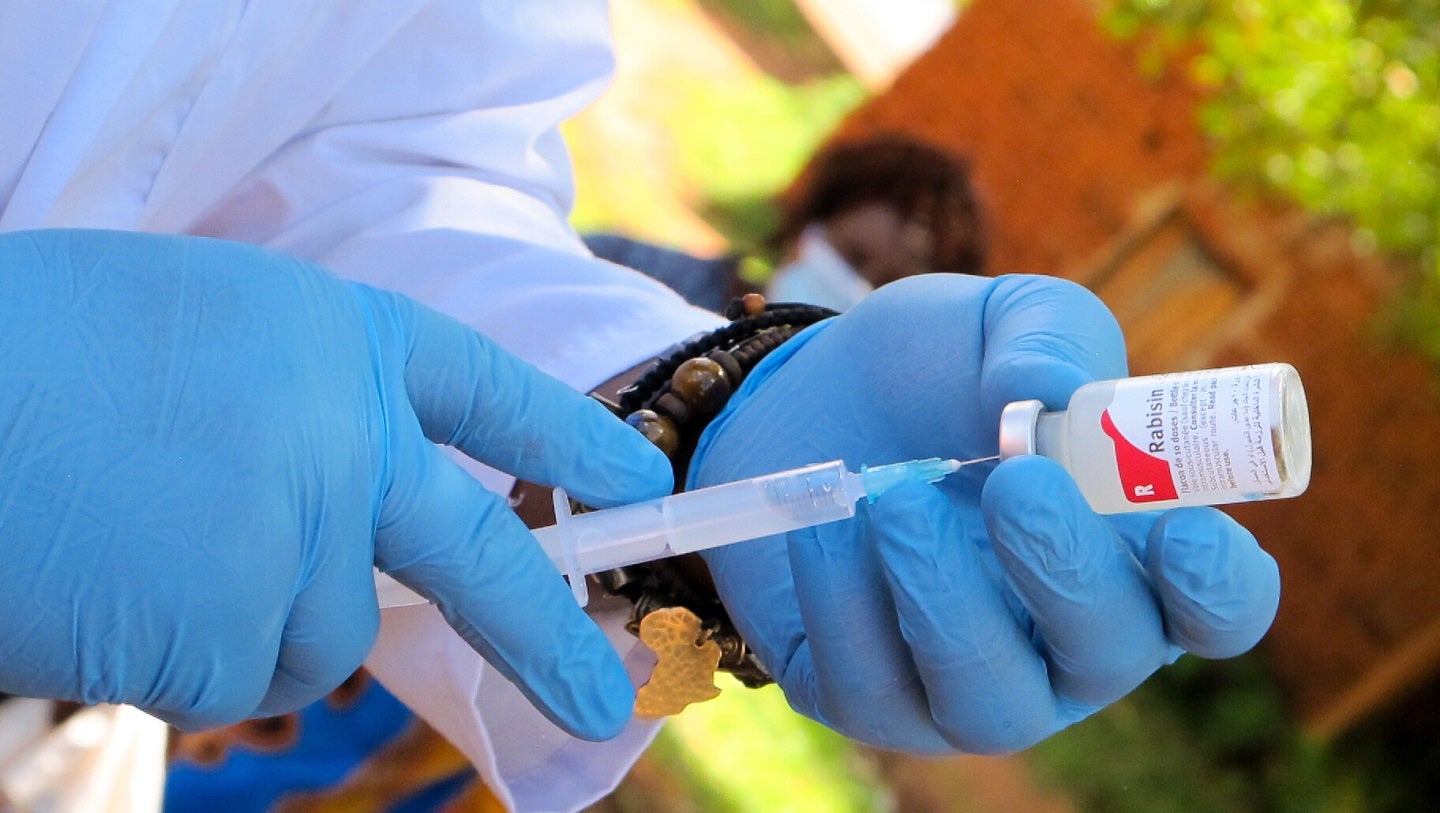
The Drug Regulatory Authority of Pakistan (DRAP) has granted approval to YS Biopharma to initiate a Phase III clinical trial of the PIKA rabies vaccine in Pakistan.
The study will evaluate the immunogenicity and safety of the vaccine in preventing rabies infection.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Developed using YS Biopharma’s proprietary PIKA adjuvant technology, the vaccine is said to accelerate immunity while providing a higher immune response.
Three clinical trials of PIKA including Phase I and Phase II were conducted in Singapore and a Phase I study in China. All three trials have shown that the PIKA rabies vaccine is safe, tolerable, and immunogenic.
YS Biopharma chief medical officer Dr Zenaida Mojares said: “We are delighted to receive approval from DRAP to conduct the PIKA Rabies Vaccine Phase III clinical trial in Pakistan.
“This approval marks a significant milestone in the development of our PIKA Rabies Vaccine as we continue to expand our clinical trial programme globally.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“Our progress enables us to advance towards our mission of providing innovative and efficacious vaccines in the fight against a vaccine-preventable rabies disease, with an almost 100% case fatality rate.”
In addition to Pakistan, the company has received approval to conduct a multi-centre, multi-country Phase III study in Singapore, and the Philippines this year.
An estimated 4,500 healthy volunteers without exposure to animal bites are anticipated to participate in Phase III clinical trials.
The company is also planning to conduct more advanced clinical trials of the PIKA Rabies Vaccine in China.
It intends to submit the New Drug Application or Biologics License Application to regulatory authorities in China and countries in Asia and other continents.





