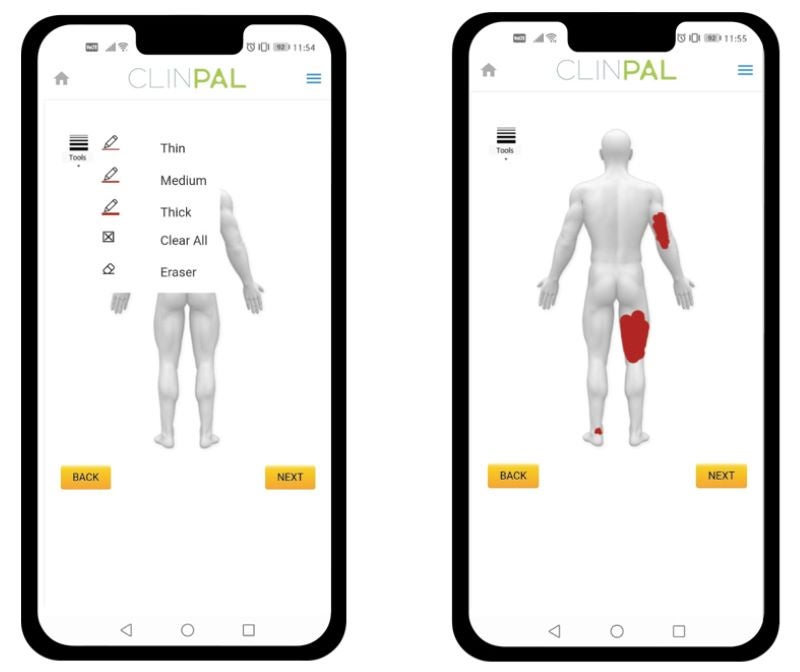
eClinicalHealth has created a digitised form of the SAPASI instrument, enabling researchers in monitoring and recording moderate-to-severe plaque psoriasis in patients enrolled in the clinical trials.
The digitised version was developed by the company in partnership with the Department of Dermatology at Wake Forest University School of Medicine in the US.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
This novel SAPASI ePRO tool, which is part of eClinicalHealth’s decentralised clinical trial (DCT) research platform Clinpal, aids patients on any device to indicate where their psoriasis is present on a digital body map at time intervals mentioned under the protocol.
It also aids in answering various prompts linked to psoriasis lesion severity.
This information is incorporated into a data model that researchers could use and track the effectiveness of a therapy.
The digital map uses the Self-administered Psoriasis Area Severity Index (SAPASI), an established diagnostic tool of Wake Forest.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe library of ePRO tools was expanded with the implementation of SAPASI on the Clinpal platform.
Clinpal platform offers a rapid trial set-up, a subject-friendly design and automatically computes the SAPSI score using an in-built compliance reminder mechanism to guarantee precise data delivery.
Furthermore, it integrates seamlessly into the current Clinpal app without needing custom development.
The company noted that the digital map feature, part of the Capture module of the Clinpal platform, is one among the seven modules that constitute the complete DCT platform.
Each module targets a vital role in trials, such as subject recruitment, successful self-registration and pre-screening expertise, intentional communication to eliminate subject noncompliance before trial commencement and ePRO tools, such as the latest digital SAPASI instrument.
eClinicalHealth CEO and board chairman Karl Landert said: “We’re excited about this evolution of our ePRO tools and pushing the Clinpal platform into new areas.
“The new normal world has revealed the benefits of a telemedicine ecosystem for end-users. As DCTs gain traction, our aim is to further scale and simplify patient engagement.”
In September last year, eClinicalHealth and Larsen & Toubro Infotech entered a strategic collaboration for decentralising clinical trials for quicker outcomes.





