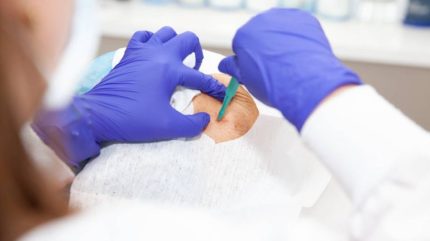
The US Food and Drug Administration (FDA) has kicked off the year by publishing draft guidance when it comes to the taking of skin biopsies for clinical trials in a bid to mitigate risk and standardise a well-used practice in the oncology space.
A skin biopsy is a procedure that involves removing a small cross-section of a patient’s skin for direct testing with the aim of identifying the symptoms and signs of various types of cancer. Given the invasive nature of the procedure, the FDA has now finalised its guidance adding considerations for determining if a tissue biopsy should be required or optional for both adults and children participating in clinical trials.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
At the same time, the new draft guidance addresses risk and benefit considerations specific to tissue biopsies conducted in clinical trials involving children. It also reinforces the importance of obtaining informed consent from participants, including parental or guardian permission for children as well as assent from children.
Richard Pazdur, director of the FDA’s Oncology Center of Excellence, said: “Tissue biopsies in clinical trials are often needed to determine eligibility or to understand the effect of the medical product being studied in the trial. This new draft guidance builds on the agency’s ongoing efforts to enhance clinical trials by providing recommendations to improve participant safety and further clinical research.”
The guidance entitled “Considerations for Including Tissue Biopsies in Clinical Trials: Guidance for Industry, Investigators, Institutions, and IRBs” was developed by the FDA’s Oncology Center of Excellence alongside the Office of the Chief Medical Officer and Center for Drug Evaluation and other FDA.
An excerpt from the guidance reads: “Sponsors should consider, among other things, whether the risks of the biopsies and any other risks of the trial are reasonable in relation to the anticipated benefits, if any, to the participants, and the importance of the knowledge that may be expected to result. For the purposes of this guidance, ‘required’ biopsies refer to those that are specified in the clinical protocol as a condition of trial participation.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataNow, the governmental body has called on industry stakeholders for feedback on the new guidance with the window for comment set to close 60 days after publication, ending on 10 March.
Elsewhere in the regulatory space, last year the FDA released its draft guidance on the topic of improving diversity in clinical trials with the aim of gaining more universally applicable results from research. Later that same year the body similarly issued draft guidance on protocol deviations in the case of Clinical Investigations for drugs, biological products, and devices.
Elsewhere in the oncology space, a Munich conference has seen sponsors and clinical research organisations told not to allow assumptions and poor record-keeping to put oncology trial participants at risk.





