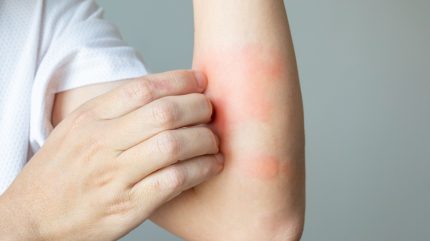
Galderma has reported that the randomised Phase III OLYMPIA 1 trial, which evaluated the nemolizumab monotherapy in adult subjects with moderate-to-severe prurigo nodularis (PN), has achieved both primary and all key secondary goals.
The double-blind, placebo-controlled, randomised study evaluated the monoclonal antibody’s efficacy and safety against placebo over a 24-week treatment period. It included 286 subjects aged at least 18 years with moderate-to-severe PN.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Findings showed that this therapy as a single agent, without the background topical corticosteroids or topical calcineurin inhibitors usage, led to statistically significant and clinically meaningful improvements in both primary endpoints versus placebo.
Subjects treated with nemolizumab reported a minimum of four-point itch intensity improvement and a higher rate of skin lesion clearance or near-clearance after 16 weeks of treatment. These results were compared to the placebo group, with 58.4% versus 16.7% and 26.3% versus 7.3%, respectively, achieving the outcomes.
The trial also confirmed rapid responses to sleep disturbance and itch as early as week four.
Notably, over six times as many subjects in the therapy arm reported itch response, and more than 20 times as many reached a peak-pruritus numerical rating scale (PP-NRS) score of less than two compared to placebo.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataGALDERMA R&D global head Baldo Scassellati Sforzolini said: “These results, alongside the OLYMPIA 2 trial data, formed the basis of nemolizumab’s recent US Food and Drug Administration approval for the treatment of adults with PN.
“They demonstrate the potential of this treatment to rapidly and significantly provide relief from the most burdensome symptom for people with PN – itch. We are committed to bringing this treatment option to patients in other parts of the world as soon as possible.”
The US Food and Drug Administration has approved nemolizumab under the name Nemluvio for this indication. It has also accepted the therapy’s biologics licence application (BLA) for review to treat moderate-to-severe atopic dermatitis in adolescents and adults, and the decision is anticipated by the end of 2024.
Initially developed by Chugai Pharmaceutical, nemolizumab was licensed to Galderma in 2016.
Last month, Galderma reported positive data from a Phase III trial of RelabotulinumtoxinA (Relfydess) for moderate-to-severe frown lines and crow’s feet treatment.



