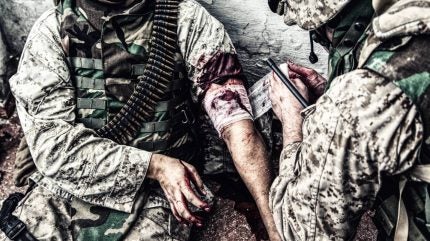
Humacyte’s acellular tissue-engineered vessel (ATEV), designed for use in extreme situations where civilians or soldiers have been injured, is better at saving limbs and staving off infection than the current standard of care.
Published in the Journal of the American Medical Association: Surgery, the entry comprises two previous non-randomised clinical trials examining the company’s ATEV system in the repair of arterial injuries with participants coming from trauma centres in the US, Israel and five frontline hospitals in Ukraine.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
All patients had some form of vascular injury with no opportunity for revascularization, putting them at risk of infection or losing a limb. The first of those studies, known as V005 (NCT03005418), was a Phase II/III, single-arm study conducted in the US and Israel examining patients with injuries resulting from gunshots, workplace injuries, car accidents, or other traumatic events. The second, V017 (NCT03183245), was a Phase III study which evaluated patient outcomes from a humanitarian program working alongside patients with wartime injuries in Ukraine.
Results found that ATEV demonstrated higher patency, a measure of the durability of vascular reconstructions and clear vessels than currently used synthetic graft treatments. Results showed a 30-day secondary patency rate of 91.5% for the extremity patients compared to 78.9% historically reported for synthetic grafts.
Rishi Kundi, chief of vascular and endovascular trauma at the University of Maryland’s R Adams Cowley Shock Trauma Center, said: “I believe that the ATEV will revolutionise vascular trauma care and be profoundly beneficial to our patients. Based on my personal experience so far, the ATEV will allow reconstruction that is currently impracticable because of contamination or infection; moreover, it will make reconstruction that we are now forced to perform with prosthetic or even biologic grafts more successful. I am excited about the promise that the ATEV holds for the long-term experience and outcomes of our patients.”
The biotech argues that one of the core benefits of its engineered tissue is its ability to be used immediately off the shelf as harvesting a vein from a trauma patient requires critical surgical time. Now Humacyte says it is applying for a Biologics License Application (BLA) for the ATEV from the US Food and Drug Administration (FDA).

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataElsewhere in the field of emergency trauma care, Arizona-based diagnostic firm Maui Imaging has emerged from stealth, revealing a $4m deal with the US Department of Defense (DOD) to support its trauma response for the military. Meanwhile, SolasCure has announced that it will collaborate with the US Army Institute of Surgical Research (USAISR), to evaluate the use of its investigational wound gel, Aurase.





