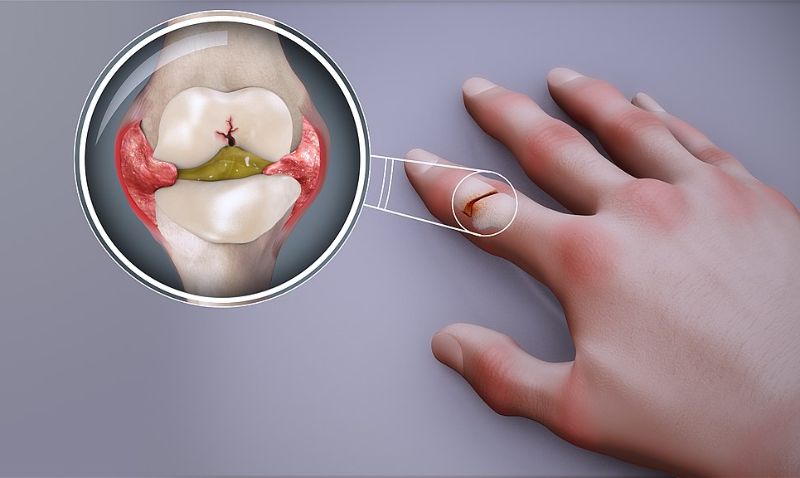
Biopharmaceutical firm I-Mab has commenced dosing patients in a Phase Ib clinical trial of plonmarlimab (TJM2) for the treatment of rheumatoid arthritis (RA) in China.
Plonmarlimab is a humanised immunoglobulin G1 (IgG1) antibody designed to act on the cytokine granulocyte-macrophage colony-stimulating factor (GM-CSF), which is associated with autoimmune and inflammatory diseases.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
GM-CSF neutralisation can suppress inflammatory responses and is expected to be clinically beneficially to patients with autoimmune disorders such as RA.
I-Mab CEO Dr Joan Shen said: “Plonmarlimab is the first antibody of its class entering clinical trials in China. We believe it has the great potential to become a new treatment option as a disease-modifying anti-rheumatic agent.
“Our intention is to achieve proof of concept in RA and expand to broad autoimmune diseases with unmet needs.”
The multi-centre, double-blind, placebo-controlled Phase Ib trial will assess the safety, tolerability, pharmacokinetics, pharmacodynamics and immunogenicity of the drug in 63 patients with RA.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataParticipants will be given a single dose or multiple doses of the therapy for up to eight weeks. The company has completed a first-in-human single ascending dose trial of plonmarlimab in healthy volunteers in the US.
Trial lead investigator Zhan-Guo Li said: “Studies have shown that GM-CSF has a profound role in modulating immune response and suppressing autoimmune diseases.
“We are eager to further investigate and characterise the safety and efficacy profile of plonmarlimab in treating patients with RA, a disease that continues to afflict five million people in China today.”
The company is currently conducting a Phase II trial to treat patients with Covid-19-related cytokine release syndrome in the US.
I-Mab filed an investigational new drug (IND) application with South Korea’s Ministry of Food and Drug Safety (MFDS) to perform a trial of TJM2 to treat cytokine storm in patients with severe or critical Covid-19.



