Incendia Therapeutics has enrolled the first subject in a Phase Ic clinical trial of PRTH-101, a novel DDR1 inhibitor aimed at treating advanced or metastatic solid tumours.
The open-label trial will examine the safety, tolerability, and anti-tumour activity of PRTH-101, both as a standalone therapy and in combination with pembrolizumab.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
This study anticipates enrolling up to 270 patients in the US.
It is designed to escalate doses and expand upon determining the optimal dosing regimens for future Phase II clinical programmes.
The trial will also explore DDR1 and related proteins as potential biomarkers for patient responses.
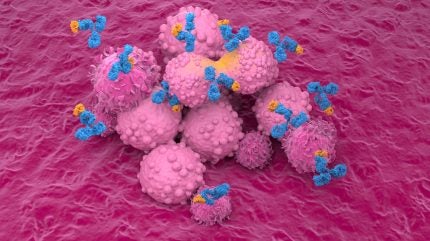
PRTH-101 is an antibody that targets DDR1, a protein involved in tumour growth.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIt is said to have shown promise in preclinical studies, demonstrating the ability to disrupt tumour defences and enhance the effectiveness of checkpoint inhibitors.
Incendia Therapeutics is at the forefront of developing therapies that alter the tumour microenvironment.
Incendia Therapeutics Development and Operations senior vice-president Irena Webster said: “In our Phase Ia and Ib studies, we were able to select an optimal dose and identify potential biomarkers to carry forward.
“The data from this study will inform the design of the Phase II/III programme, for which planning and global feasibility are currently underway.”
Incendia Therapeutics CMO Dr Joseph Paul Eder said: “Incendia, with its outstanding team of investigators and investigative sites, has determined a recommended Phase II dose of PRTH 101.
“PRTH 101 has shown consistent safety as a single agent and in combination with pembrolizumab. We will now focus on tumour types that have demonstrated signs of clinical activity and patient benefits.”





