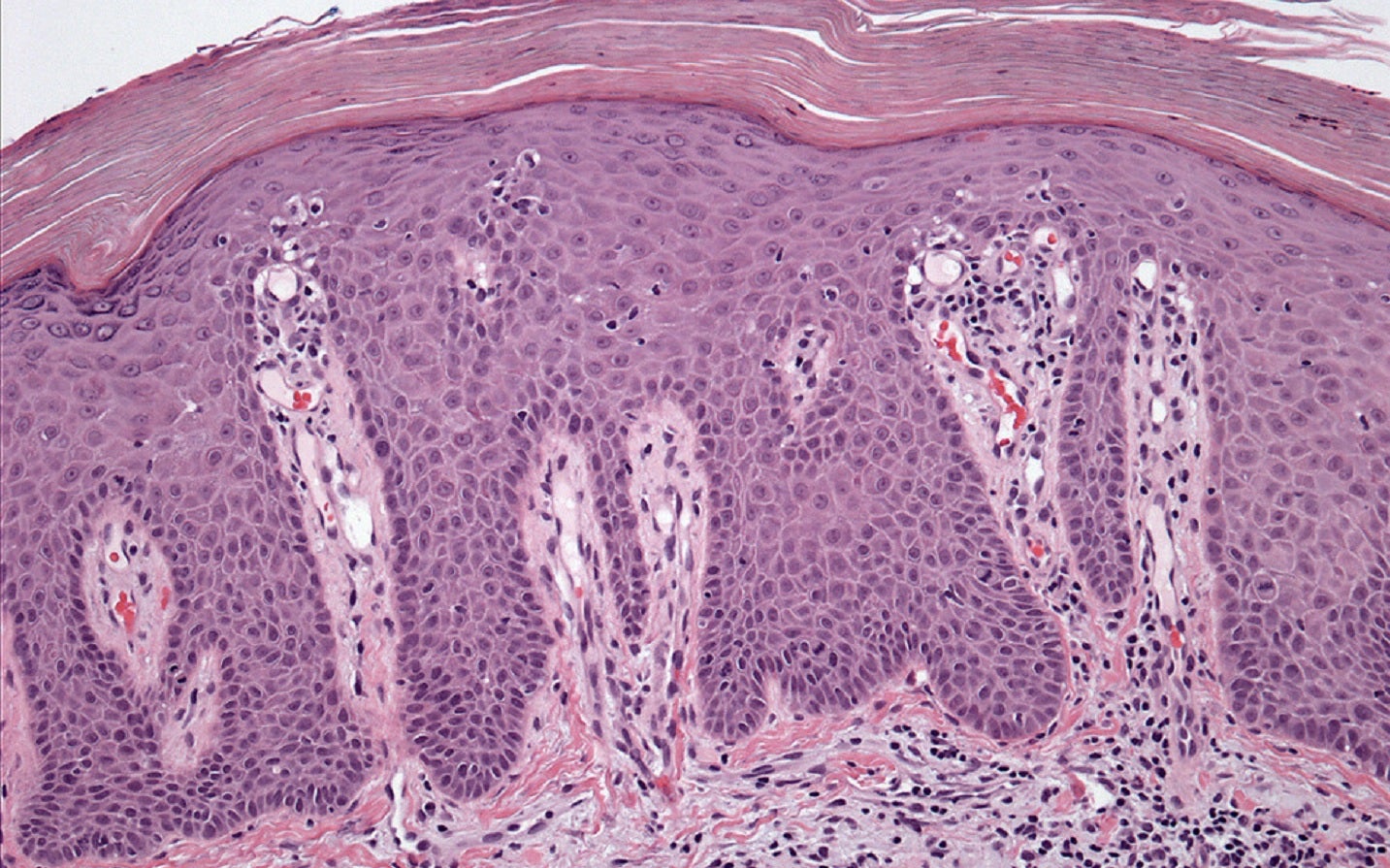
Innovent Biologics Group has dosed the first participant in the Phase III CLEAR clinical trial of the anti-interleukin 23p19 subunit (IL23p19) antibody injection, picankibart, in moderate-to-severe plaque psoriasis patients.
The placebo-controlled, multicentre, double-blind Phase III clinical trial has been designed for assessing picankibart’s safety and efficacy in these patients.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Nearly 500 moderate to severe plaque psoriasis patients are planned to be enrolled in the trial.
Participants who meet the study eligibility criteria will be randomised into a 2:2:1 ratio to picankibart group 1, picankibart group 2, or a placebo group after screening for four weeks.
The company expects the research period to be 68 weeks.
CLEAR is the first Phase III study of IL-23 class innovative drugs in China.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataInnovent Biologics Group Clinical Development vice-president Dr Qian Lei said: “The new generation of drugs targeting IL-23 has shown excellent efficacy and good safety, especially compared with anti-IL-17 monoclonal antibody drugs.
“Picankibart, independently developed by Innovent, is the first anti-IL23p19 antibody drug which has completed Phase II clinical study in Chinese patients with moderate-to-severe plaque psoriasis, and has obtained good efficacy and safety results.
“It also showed the obvious advantages of long-dose interval and long-term efficacy. At week 52, 66.7%~86.0% of the subjects who received picankibart achieving PASI90, 81.6%~88.0% of the subjects achieved PASI 75, and in one of the groups received picankibart, about 48.0 % of subjects achieved complete skin lesions clearance (PASI 100).”
Picankibart works by specifically binding to IL-23p19 subunit, then preventing IL-23 from binding to cell surface receptors. This results in IL-23 receptor-mediated signalling pathway inhibition.





