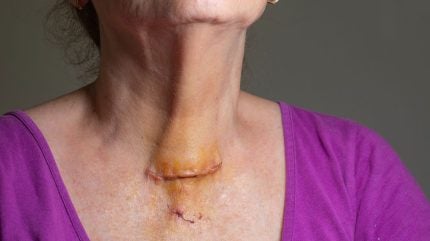
Innovo Therapeutics has reported promising results from its Phase II clinical trial of INV-001, a treatment designed for post-thyroidectomy scar reduction.
The trial was conducted at four general hospitals, including Severance Hospital in Seoul. It assessed 77 patients with wounds larger than 3cm following thyroidectomy.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Participants were randomly assigned to apply INV-001 twice daily for 12 weeks, and efficacy was evaluated using the Patient and Observer Scar Assessment Scale (POSAS) at week 12.
According to the findings, the study demonstrated a significant reduction in scar size with the application of INV-001.
The results confirmed the safety and tolerability of INV-001, with no serious adverse events reported for both low (0.2%) and high (2%) doses.
Most notably, a 24.5% reduction in scars was observed in the high-dose group at week 12, a statistically significant difference compared to the placebo.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe primary efficacy endpoint of the trial was the Overall Opinion score on the POSAS Observer scale at 12 weeks.
A trend of lower scores, indicating scar improvement, was observed in each treatment group compared to the placebo. The high-dose group, in particular, showed a statistically significant reduction in scores from baseline to 12 weeks.
Secondary efficacy evaluations revealed a trend of scar improvement at three and six weeks, though no statistically significant differences were noted until the high-dose group showed a gradual improvement effect over time.
Pharmacokinetic analysis results indicated that the average blood concentration of INV-001 in the high-dose group was below 3.6ng/mL at three weeks, maintaining similar levels at six and 12 weeks.
Safety evaluations revealed that all adverse drug reactions were predictable and related to the application site, with no serious adverse events or serious adverse drug reactions reported.
Innovo Therapeutics founder and CEO Dr Hee Dong Park said that the clinical trial confirmed INV-001’s safety and efficacy.





