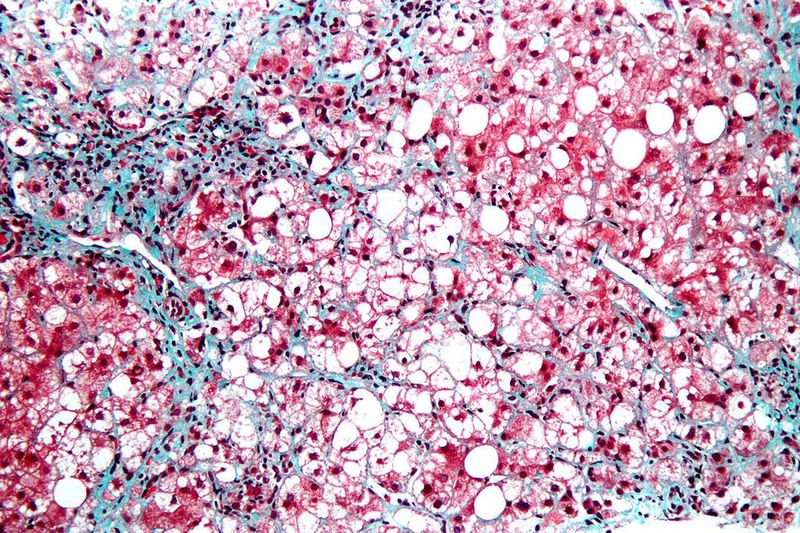
Intercept Pharmaceuticals has reported positive top-line results from the pivotal Phase III REGENERATE clinical trial of obeticholic acid (OCA) in patients with liver fibrosis caused due to non-alcoholic steatohepatitis (NASH).
Primary efficacy analysis showed fibrosis improvement with no worsening of NASH, when treated with once-daily 25mg OCA, at 18 months. The analysis was carried out in a total of 931 patients.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The trial met the primary endpoint of NASH resolution with no worsening of liver fibrosis in more patients in OCA arms, compared to placebo, but did not achieve statistical significance.
An additional full efficacy analysis at 18 months included an exploratory cohort of 287 participants with stage I liver fibrosis, additional risk factors and an increased risk of progression to cirrhosis.
Safety analysis at 18 months involved 1,968 patients who received at least one dose of OCA or placebo. Adverse events were found to be generally mild to moderate in severity, with the most common being dose-related pruritus.
Intercept Pharmaceuticals president and CEO Mark Pruzansk said: “The topline REGENERATE data we are reporting today support our belief that OCA will become the first approved medicine for those living with liver fibrosis due to NASH.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataREGENERATE is a randomised, double-blind, placebo-controlled, multi-centre trial being conducted to evaluate the safety and efficacy of OCA on liver-related clinical outcomes.
The trial is designed to enrol more than 2,000 adult NASH patients with stage II and III fibrosis at 339 sites globally.
Following the interim analysis, the trial will be continued through clinical outcomes to confirm clinical benefit. The final assessment will track OCA’s effect on mortality and liver-related clinical outcomes, along with its long-term safety.
Based on the top-line data, the company is planning to seek approval in the US and Europe in the second half of this year.





