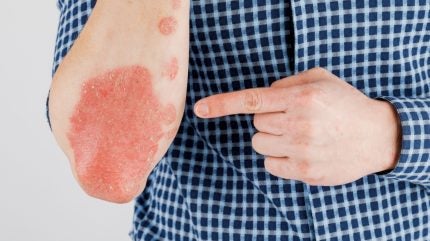
Johnson & Johnson (J&J) has reported data from the Phase III ICONIC-LEAD trial’s subgroup analysis evaluating icotrokinra in treating moderate-to-severe plaque psoriasis (PsO).
The data revealed that adolescents who were administered the therapy once a day achieved ‘higher’ rates of clear or almost clear skin at the 16-week mark compared to those receiving the placebo, without any safety signals detected.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Icotrokinra is an investigational oral peptide said to selectively inhibit the interleukin-23 (IL-23) receptor, and it is being evaluated in individuals aged 12 and above with this condition.
The randomised controlled trial aims to assess icotrokinra’s safety and efficacy compared to a placebo in 684 subjects, with 66 adolescent patients.
This study claims to be the first Phase III registrational trial to evaluate a systemic therapy’s safety and efficacy in both adolescents and adults concurrently.
The trial’s co-primary endpoints are an Investigator’s Global Assessment (IGA) score of 0/1, with a minimum of a two-grade improvement, and a higher efficiency bar using the Psoriasis Area and Severity Index (PASI 90).

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataAt week 16, 84.1% of adolescents treated with the therapy achieved an IGA score of 0/1, and 70.5% reached a PASI 90 response, versus 27.3% and 13.6% on placebo, respectively.
These response rates continued to enhance through week 24, in which 86.4% and 88.6% of adolescent subjects achieved IGA 0/1 and PASI 90 responses, respectively.
In addition, at week 24, 75% and 63.6% of adolescent subjects achieved IGA 0 and PASI 100 scores, respectively.
The safety profile of icotrokinra was demonstrated to be ‘favourable’, according to the company.
The licence and collaboration agreement signed in 2017 between Protagonist Therapeutics and Janssen Biotech allowed the two companies to jointly discover and develop next-generation compounds, ultimately leading to icotrokinra.
J&J innovative medicine immunodermatology disease area lead and vice-president Liza O’Dowd said: “Adolescents living with moderate to severe plaque psoriasis shouldn’t have to wait for effective treatment options that have the potential to deliver completely clear skin, which is the driving force for studying this younger population as part of the pivotal ICONIC programme.”
Icotrokinra is currently under investigation in the pivotal Phase III ICONIC clinical development programme for moderate-to-severe PsO and active psoriatic arthritis, as well as the Phase IIb ANTHEM-UC study for moderately to severely active ulcerative colitis.





