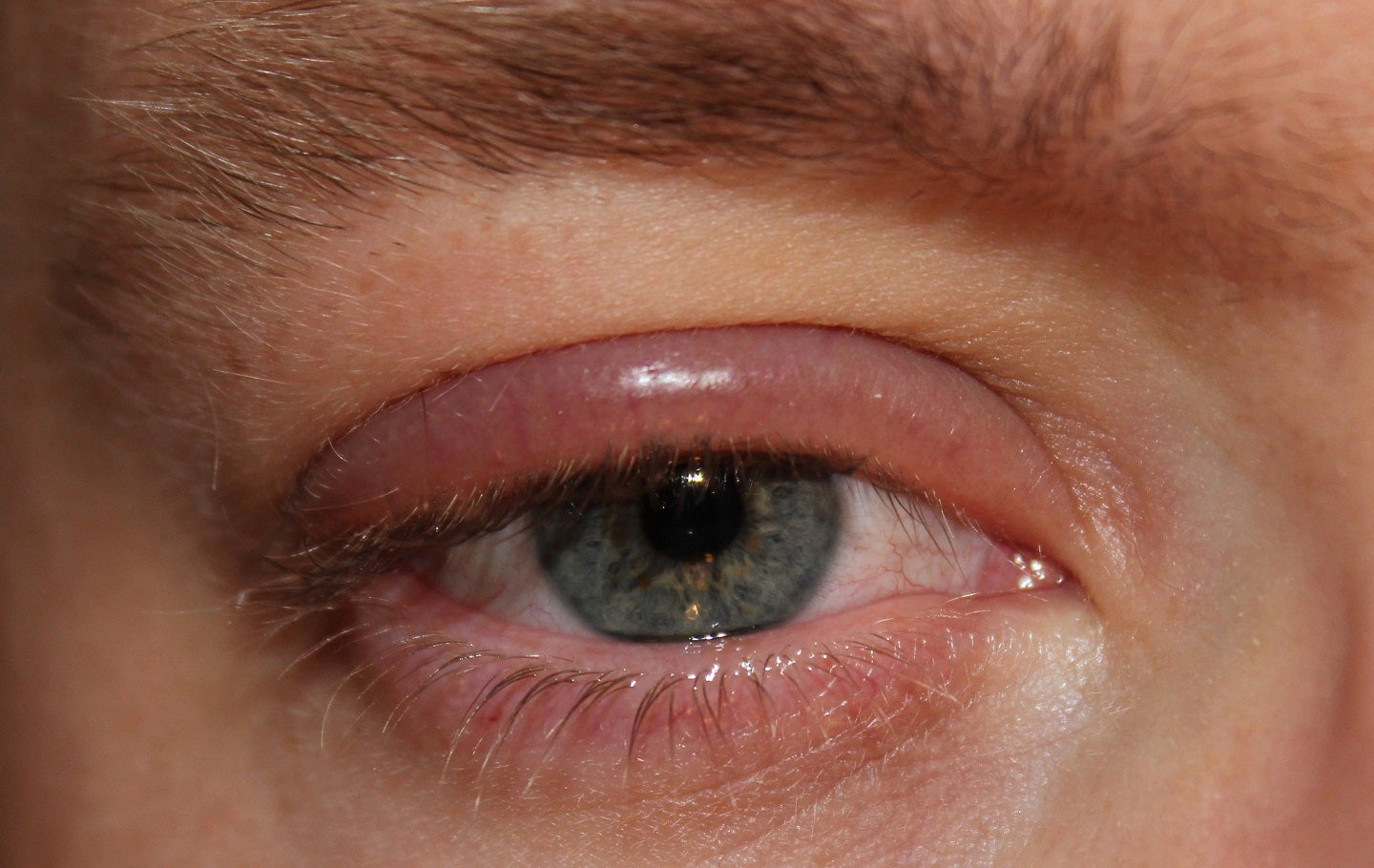
LianBio has completed enrolment in the Phase III LIBRA clinical trial of TP-03 (lotilaner ophthalmic solution, 0.25%) in Demodex blepharitis patients in China.
The randomised, multicentre, double-blind, vehicle-controlled registrational trial has been designed to assess the safety and efficacy of TP-03.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It includes a single-arm, open-label pharmacokinetics sub-study to assess systemic TP-03 in whole blood after topical ocular administration.
Mite eradication with a mite density of zero mites per lash and complete collarette cure with a collarette score of zero at day 43 are the trial’s co-primary endpoints.
The composite cure of collarette and erythema at day 43 are some of the secondary endpoints of the trial.
The topline results from the trial are expected to be reported in the fourth quarter of this year.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataLianBio CEO Yizhe Wang said: “LianBio is committed to bringing a safe and effective treatment option to the tens of millions of patients living with Demodex blepharitis in China.
“Although patients with Demodex blepharitis experience a significant impact to the quality of life, today there are no available treatments that address the underlying cause of their disease.”
An investigational therapeutic, TP-03 has been designed to mitigate the signs and symptoms of Demodex blepharitis.
It targets and eradicates the root cause of the disease, Demodex mite infestation.
The company noted that the therapy effectively resolved Demodex blepharitis and met all primary and secondary endpoints in two pivotal Phase III trials, which were conducted in the US by its partner Tarsus Pharmaceuticals.
It in-licensed the rights from Tarsus to develop and commercialise TP-03 in Mainland China, Taiwan, Macau, and Hong Kong.





