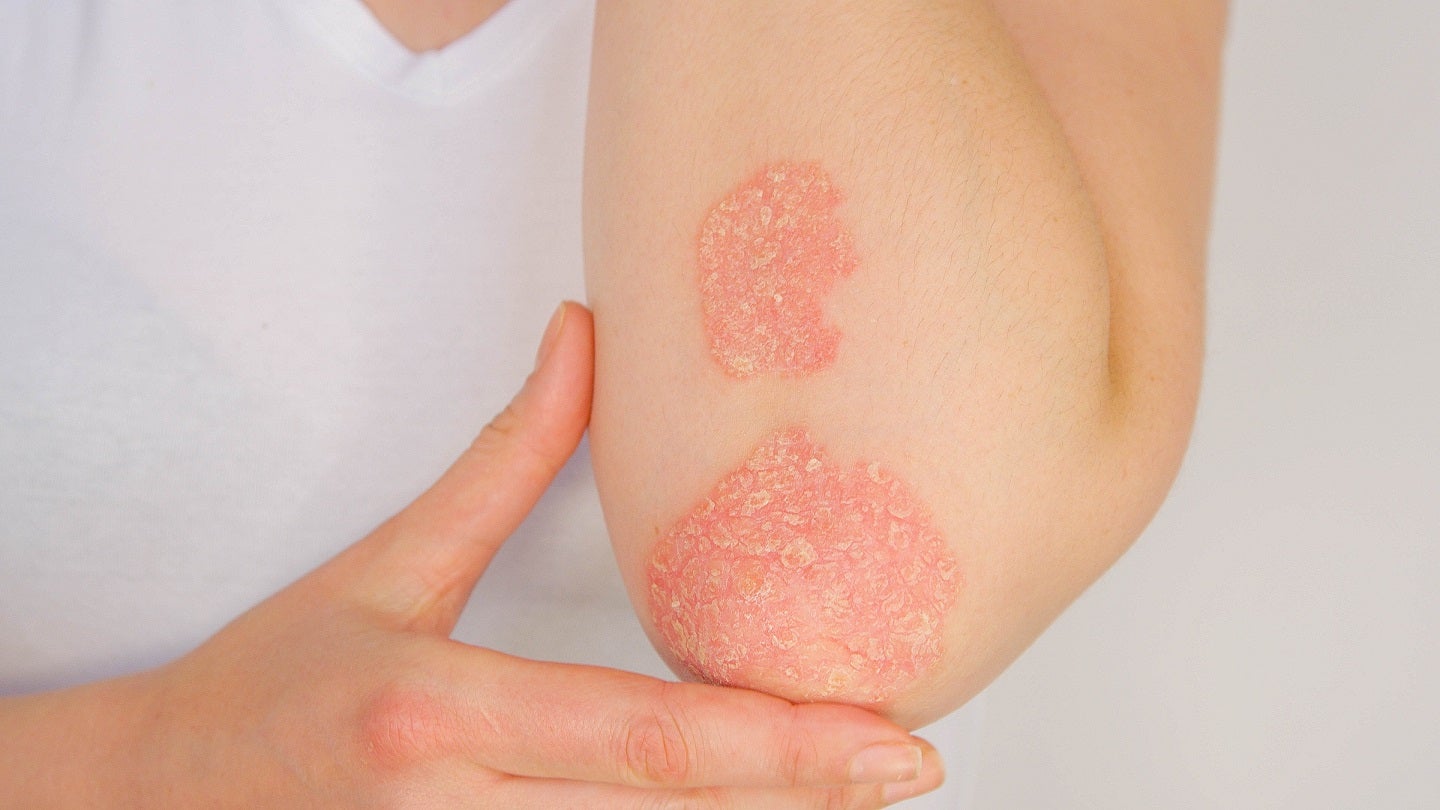
Lynk Pharmaceuticals has dosed the first cohort of patients in a Phase Ib clinical trial of LNK01004 ointment to treat psoriasis, a chronic inflammatory skin disease.
The study will evaluate preliminary efficacy of LNK01004 in Chinese patients with mild to moderate plaque psoriasis.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It will also assess the safety, tolerability and pharmacokinetic properties of the topical ointment.
Lynk Pharmaceuticals chief development officer Dr Henry Wu said: “LNK01004 has already shown good efficacy and safety in preclinical animal studies.”
Characterised by scaly erythematous or plaque lesions, psoriasis is an immune-mediated, relapsing skin disease that can be local or widely distributed.
Lifelong treatment is required for psoriasis patients to prevent complications from developing, thereby maintaining their quality of life.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataLynk Pharmaceuticals founder and CEO Dr Zhao-Kui (ZK) Wan said: “As a third generation JAK inhibitor, LNK01004 can effectively inhibit psoriasis-related cytokine-induced JAK/STAT signaling pathways in skin tissues by topical application.
“Additionally, LNK01004 has the advantage of extremely low exposure in the blood system to potentially avoid safety concerns due to systemic exposure.
“In addition to the highly selective JAK1 inhibitor LNK01001, we have also assembled a promising pipeline consisting of multiple third-generation, tissue-restricted soft pan JAK inhibitors in clinical trials including the GI-restricted LNK01003 for UC which is currently in Phase IIa.”
Last week, Lynk reported that the Phase II study of LNK01001 for rheumatoid arthritis treatment demonstrated statistically significant differences in efficacy against placebo in primary and key secondary efficacy endpoints. It is actively seeking a partner for developing LNK01001 in markets worldwide.





