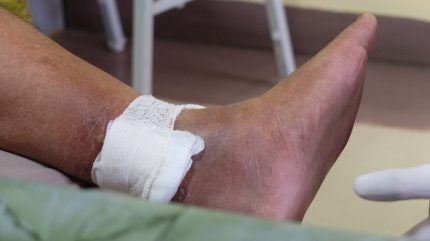
MediWound has published Phase II trial data showing that its wound debridement therapy, EscharEx, is superior at treating venous leg ulcers than other non-surgical standard-of-care solutions.
The Phase II ChronicEx trial (NCT03588130) data was published in the eClinicalMedicine journal, part of the Lancet Discovery Science.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
MediWound aims to start a Phase III trial evaluating EscharEx as a treatment for venous leg ulcers in the second half of this year.
“Removing non-viable tissue and promoting well-vascularised granulation tissue are essential steps in wound bed preparation, which is crucial for successful wound healing,” stated Dr John C Lantis, principal investigator in the ChronEx study.
“The significant superiority of EscharEx over the current non-surgical standard of care in achieving optimal wound bed preparation could dramatically enhance healing outcomes and provide a viable alternative to surgical debridement. This will be a primary focus of the upcoming EscharEx Phase III trial in treating venous leg ulcers.”
The ChronEx study enrolled 119 patients, who received either EscharEx, a placebo gel vehicle or a non-surgical standard of care treatment such as Collagenase Santyl ointment hydrogels, medical grade honey, and non-active dressings. The trial met its primary endpoint with 63% of participants who received EscharEx achieving complete debridement, compared to 30.2% and 13.3% in the placebo and non-surgical standard of care treatments respectively.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe median time to complete debridement was nine days for EscharEx, compared to 63 days for placebo and 59 days for non-surgical standard-of-care treatments. Furthermore, the incidence of a complete cover of the wound bed with healthy granulation tissue during the daily treatment period was 50.0% for EscharEx compared to 25.6% and 10.0% for placebo and non-surgical standard-of-care treatments respectively.
EscharEx is a bioactive therapy that consists of a concentration of proteolytic enzymes. It is being developed for debridement of chronic and other hard-to-heal wounds. MediWound also plans to investigate EscharEx in another chronic wound condition, diabetic foot ulcers in the second half of 2025.
Another product in MediWound’s portfolio is NexoBrid (anacaulase-bcdb) treatment. It was approved by the US Food and Drug Administration to eliminate eschar in adults with deep partial-thickness and/or full-thickness thermal burns in January.



