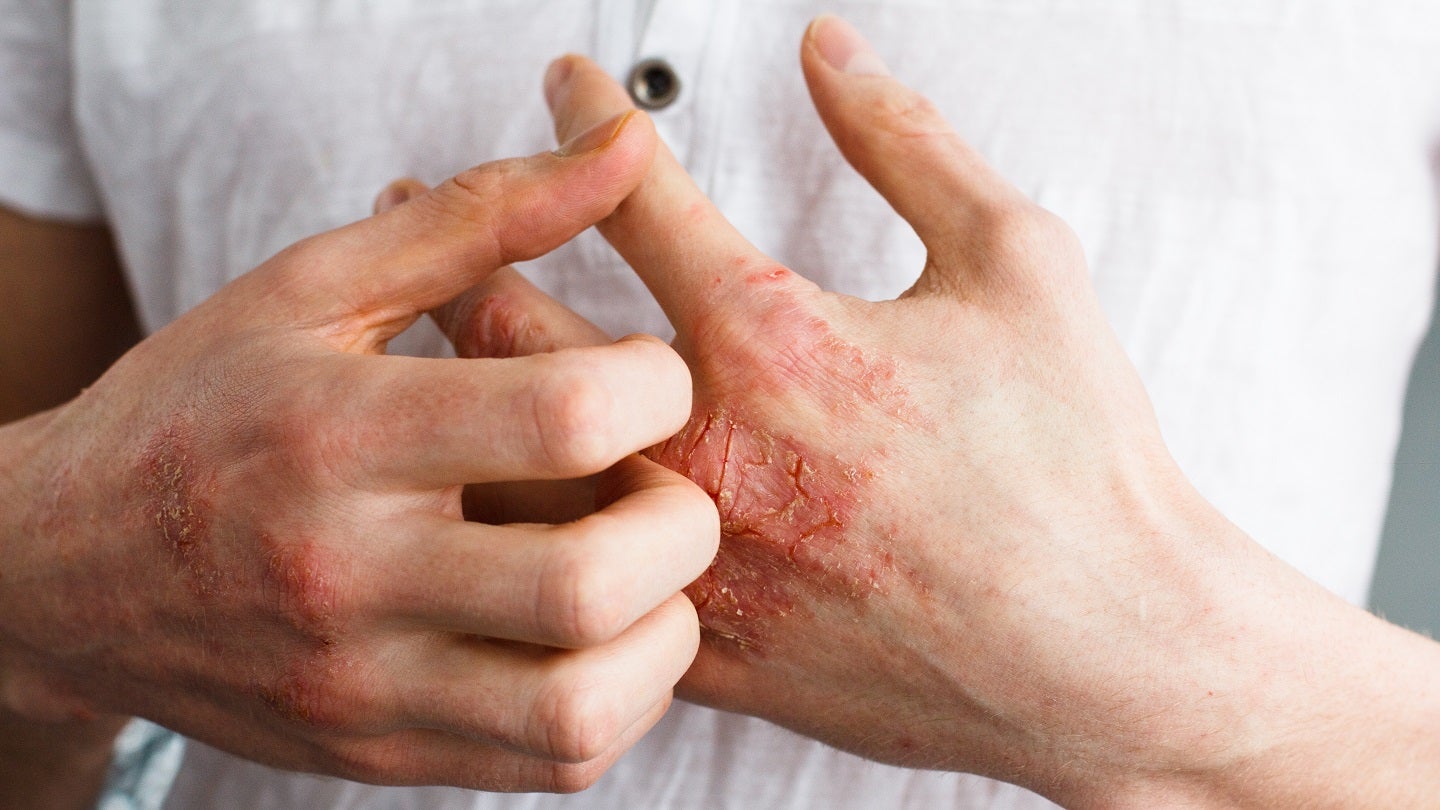
Minghui Pharmaceutical has enrolled the first patient in the Phase III clinical trial of its investigational MH004 Cream to treat mild to moderate atopic dermatitis (AD).
The placebo-controlled, double-blind, randomised study intends to assess the efficacy and safety of the topical cream of a pan-JAK inhibitor MH004 in a wider population.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Topline data from the trial is anticipated in the second half of next year.
The study is based on positive results from the proof-of-concept Phase II study that enrolled 150 adolescents aged 12 years and above and adults.
Patients with mild-to-moderate AD involving 3% ~ 20% body surface area at baseline were randomised to receive topical applications of vehicle, 0.3% or 1.0% MH004 twice-daily for four weeks.
The study met all the primary and secondary endpoints and MH004 was found to be well tolerated.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataMinghui Pharmaceutical CEO Guoqing Cao said: “The first patient enrolment marks a crucial moment in our journey to bring innovative treatments to those in need.
“Our team remains dedicated to rigorous research and development, and we are grateful to the patients, caregivers, and medical professionals who have participated in earlier stages, making this advancement possible.”
This June, Minghui dosed the first patient in its two Phase I trials of MHB036C and MHB088C antibody-drug conjugates (ADCs) for treating selected types of advanced or metastatic solid tumours.



