US-based biopharmaceutical firm FibroGen and its division FibroGen China Medical Technology Development has reported positive topline results from the two Phase III clinical trials of roxadustat to treat anaemia in China.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Roxadustat is an orally administered small molecule developed for the treatment of anaemia in dialysis-dependent CKD (DD-CKD) and non-dialysis-dependent CKD (NDD-CKD) patients.
The drug is claimed to be a hypoxia-inducible factor prolyl hydroxylase inhibitor (HIF-PHI), which improves endogenous erythropoietin and iron regulation, as well as minimises hepcidin, resulting in increased erythropoiesis.
The double-blind, placebo-controlled NDD-CKD trial conducted in 151 anaemia patients indicated that roxadustat met the primary endpoint by statistically enhancing hemoglobin (Hb) levels, when compared to placebo over eight weeks.
The drug is also reported to have met the secondary end point of Hb response.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe second dialysis study in 304 patients randomised to and treated with roxadustat or epoetin alfa, showed Hb change from baseline to the Hb level averaged during the last five weeks of the 26-week treatment period.
The results indicated that adverse events were consistent with previous clinical trials of roxadustat in the CKD patients without any new or unexpected safety signals detected.
FibroGen has partnered with AstraZeneca to develop and commercialise roxadustat in markets such as China and the US, while the firm collaborated with Astellas for Europe, Japan, the Commonwealth of Independent States, the Middle East and Africa.
A roxadustat myelodysplastic syndrome (MDS) Phase II/III clinical trial application (CTA) is currently under review by the China Food and Drug Administration (CFDA), as well as a Phase III trial is being initiated in the US to treat anaemia patients with MDS.
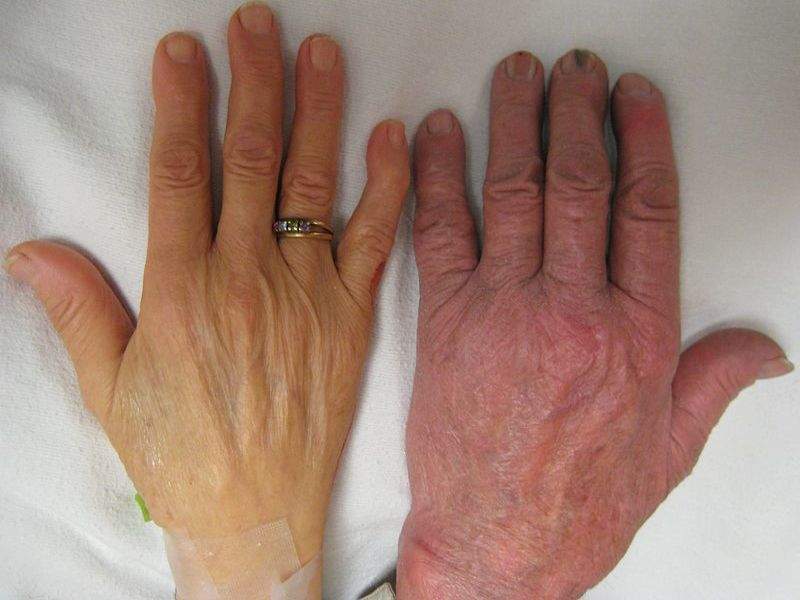
Image: The hand of a person with severe anaemia compared to one without. Photo: courtesy of James Heilman / Wikipedia.





