GlaxoSmithKline (GSK) has reported positive results from the Phase III PETIT2 study evaluating the efficacy of eltrombopag vs placebo in paediatric patients with chronic immune (idiopathic) thrombocytopenic purpura (cITP).
Ninety three subjects at 38 centres in 14 countries were enrolled in PETIT2, a two-part, double-blind, randomised placebo-controlled and open-label trial.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
In the trial, eltrombopag met its primary endpoint, achieving a statistically significant improvement in platelet counts with almost 40% of patients treated with eltrombopag attaining a consistent platelet response for six of eight weeks compared with placebo.
The trial’s primary objective was to evaluate the efficacy of eltrombopag, relative to placebo, in achieving platelet counts of =50 Gi/L among paediatric patients with previously treated cITP for at least 12 months.
Eltrombopag is marketed as Promacta in the US and as Revolade in Europe, as well as other countries worldwide.
GlaxoSmithKline head of oncology R&D Rafael Amado said: "We look forward to continuing to assess the potential of eltrombopag in these patients and to moving forward with planned regulatory submissions for a paediatric indication in cITP later this year."

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataAccording to the company, efficacy results for the trial were consistent across age cohorts, while the safety profile was consistent with the established profile for eltrombopag and no new safety concerns were observed.
Most common adverse events (AEs) occurring most frequently in the eltrombopag arm included nasopharyngitis, rhinitis, cough and respiratory tract infection.
The company said that grade 3/4 AEs occurred in 12.7% of patients treated with eltrombopag and 10.3% of patients in the placebo group, while serious AEs were reported in 8% of eltrombopag-treated patients vs 14% in the placebo arm.
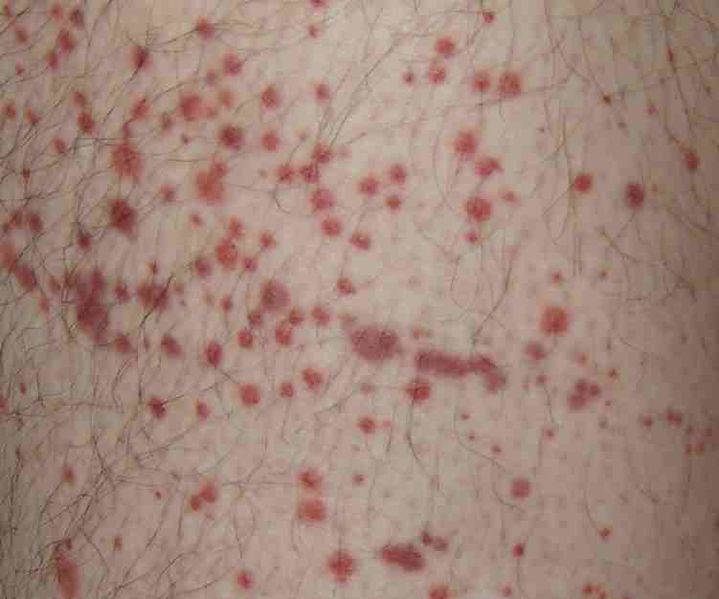
Image: Petechiae, or small bruise-like markings, may occur in ITP. Photo: courtesy of Stevenfruitsmaak.





