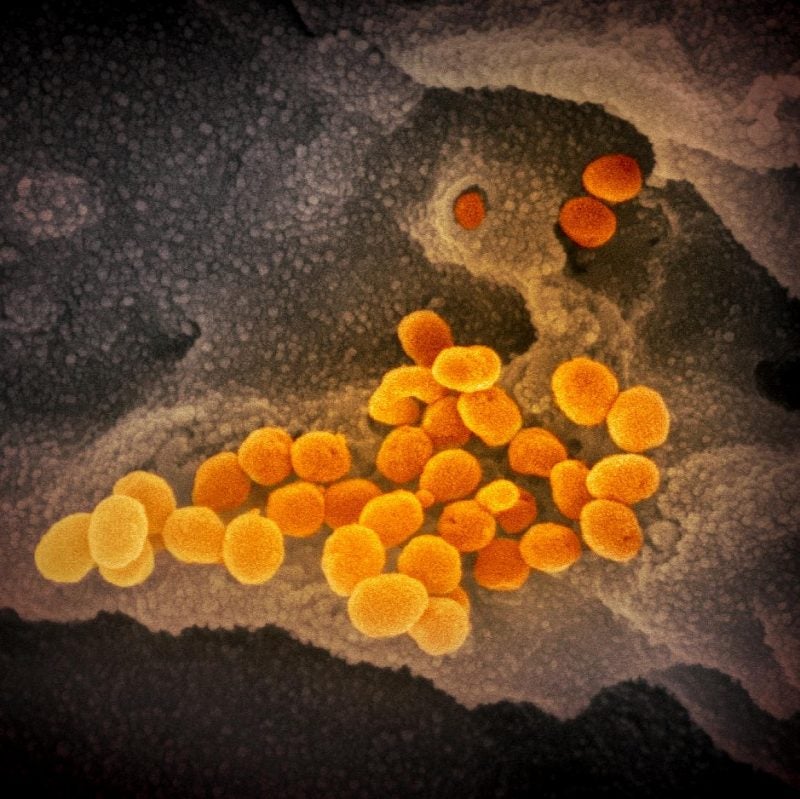
The National Institutes of Health (NIH) has reported results from a study that showed that experimental antiviral drug MK-4482 (molnupiravir and EIDD-2801) significantly lowered viral levels and disease damage in the lungs of hamsters treated for SARS-CoV-2 infection.
With NIAID funding, MK-4482 was developed by Emory University’s Drug Innovation Ventures group in Atlanta, US for treating influenza.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The oral drug is currently being jointly developed and assessed as a potential Covid-19 treatment by Merck and Ridgeback Biotherapeutics. It is in Phase II and Phase III human clinical studies.
A commonly used antiviral drug, remdesivir, which is already approved by the US Food and Drug Administration for treating Covid-19, is administered intravenously, limiting its use mainly to clinical settings.
MK-4482 was effective on administration up to 12 hours before or 12 hours after infecting the hamsters with Covid-19, researchers of the study found.
These results indicate that the drug could be used for alleviating high-risk exposures to Covid-19 and treating the disease alone or along with other agents.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataLast year, the same research team in Rocky Mountain Laboratories, part of NIH’s National Institute of Allergy and Infectious Diseases in Hamilton, Montana had developed the hamster model to imitate SARS-CoV-2 infection and mild disease in humans.
The University of Plymouth in the UK partnered on these studies.
Three groups of hamsters: a pre-infection treatment group, a post-infection treatment group, and an untreated control group, were part of the study.
The two treatment groups received an oral dose of MK-4482 every 12 hours for three days.
On concluding the study, data showed that the animals in treatment groups had 100 times fewer infectious virus and fewer lesions in their lungs as compared with the control group.
The MK-4482 doses for the study were decided based on earlier experiments in mouse models of SARS-CoV-1 and MERS-CoV, in which the drug showed effectiveness in preventing viral replication.





