US-based Noah Medical has published positive data from a clinical study demonstrating the success and safety of its Galaxy surgical robotic system.
The results were published in the Asian Pacific Society of Respirology journal and showed that the robotic platform achieved 100% successful navigation and 100% tool-in-lesion accuracy.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
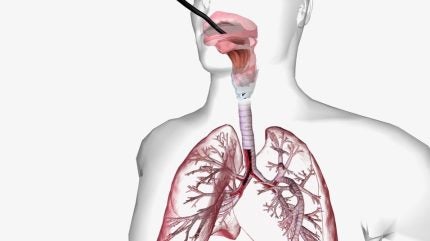
The first-in-human FRONTIER study enrolled 18 patients with moderate-risk peripheral pulmonary nodules in Sydney, Australia. The data demonstrates the Galaxy system’s capability to safely access peripheral lung nodules with bronchoscopy – a procedure used to examine and treat the inside of the lungs using a flexible tube with a camera.
Noah Medical’s Galaxy system is used to improve location accuracy and diagnosis of lung nodules. It is incorporated with TiLT+ [Tool in lesion] technology with integrated tomosynthesis and augmented fluoroscopy. It comes with a single-use disposable bronchoscope to enhance procedural workflow and has a small, compact footprint to fit in even small bronchoscopy suites.
The company received US Food and Drug Administration (FDA) clearance for the Galaxy system in March 2023. Shortly after, the medical robotics company secured $150m in a Series B funding round to scale its endoluminal robotic solutions. The oversubscribed financing round was led by Softbank Vision Fund and co-led by Prosperity7 Ventures. The first procedure was conducted in the US in May 2023, marking the platform’s commercial release.
Noah Medical’s CEO Jian Zhang said: “After years of working with leading pulmonologists to understand and address the challenges of diagnosing lung cancer with existing technologies, the Galaxy System’s groundbreaking technology makes it easier than ever to locate and diagnose this deadly disease.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataA report from GlobalData’s Medical Intelligence Center forecasts the surgical robotics market to be worth $10.3bn by 2030.
GlobalData is the parent company of Clinical Trials Arena.
In February 2024, the FDA authorised the marketing of Virtual Incision’s MIRA Surgical System, a miniRAS device, for adult colectomy procedures.
A month prior, Taiwan-based surgical robot company Brain Navi revealed that the 100th procedure was performed using its neurosurgical navigation robot, NaoTrac. The system received a CE mark in April 2021 and approval from the Taiwan Food and Drug Administration in July 2022.





