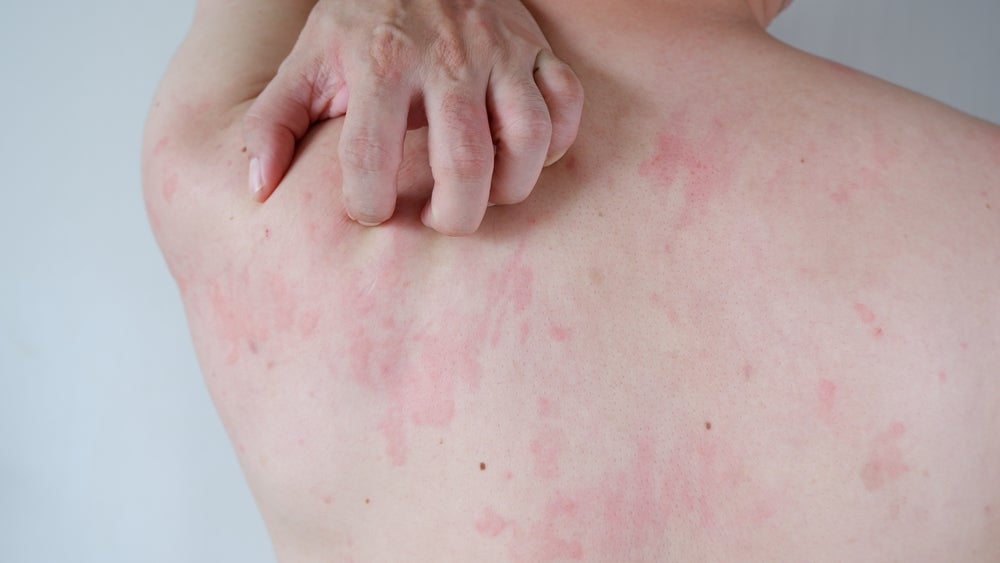
Novartis has announced that the two Phase III trials for remibrutinib have demonstrated clinical efficacy and met their primary endpoints in patients with chronic spontaneous urticaria.
Chronic spontaneous urticaria, also known as chronic hives, is a skin condition of unknown cause that causes red, raised, and itchy hives that are occasionally painful. These hives last for six weeks or more. As per Allergy UK, chronic spontaneous urticaria is not caused by allergens but by an immune response, and is managed symptomatically with anti-histamines, antipruritic, and anti-inflammatories.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Remibrutinib is a selective Bruton’s tyrosine kinase (BTK) inhibitor. It acts by stopping histamine release by blocking the BTK cascade. The release of histamine is responsible for chronic spontaneous urticaria symptoms such as itching, hives, and swelling.
The two randomised, multicentre, placebo-controlled Phase III studies (NCT05030311 and NCT05032157) demonstrated a change at 12 weeks, from baseline, on the urticaria signs and symptoms evaluation questionnaire, weekly urticaria activity score (UAS7).
Remibrutinib started showing improvement in disease activity after two weeks of therapy. It also reported a favourable safety profile in multiple conditions apart from chronic spontaneous urticaria, including multiple sclerosis, hidradenitis suppurativa, and food allergy.
“These positive top-line results from the Phase III REMIX studies confirm that remibrutinib, a highly selective BTK inhibitor, has the potential to be a first-in-class, oral treatment for people living with CSU whose symptoms are refractory despite the use of antihistamines,” said Shreeram Aradhye, chief medical officer of Novartis in a press release.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataAdditional one-year follow-up data is planned for presentation at scientific conferences in 2024. Furthermore, Novartis is planning to apply for regulatory submissions in 2024.
Sanofi’s monoclonal antibody, Dupixent (dupilumab) showed a 63% decline in the severity of itch in Phase III trials within the paediatric population (aged six years or older). Other drugs in development for chronic spontaneous urticaria include Amgen’s Tezepelumab, Allakos’ lirentelimab, and Celldex’s barzovolimab.





