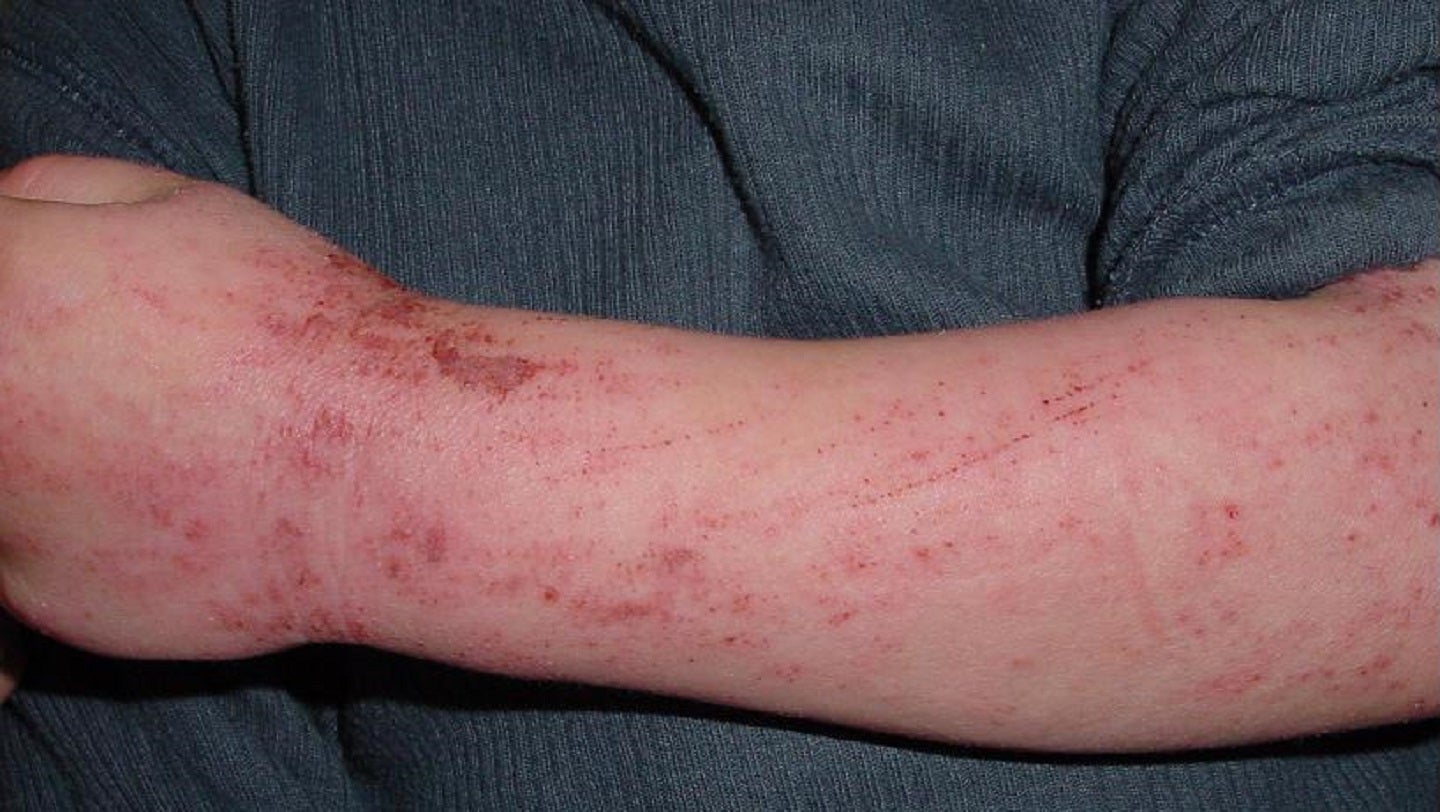
Numab Therapeutics has dosed the first subject in a Phase I clinical trial of NM26-2198, a bi-specific antibody to treat moderate-to-severe atopic dermatitis (AD).
Being conducted in collaboration with Numab’s Asia-regional partner Kaken Pharmaceutical, the study includes a combined single-ascending dose (SAD) and multiple-ascending dose (MAD) cohorts.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The SAD sub-study will include non-Asian and Japanese healthy volunteers while the MAD sub-study will enrol moderate-to-severe AD patients.
Numab Therapeutics founder and chief medical officer Peter Lichtlen said: “Based on its unique mechanism of action, we believe that NM26-2198 has the potential to deliver faster and more pronounced relief from itch than the current standard of care AD treatment.
“This effect would be notable for people suffering from AD, as we know itch is a major factor of disease burden that negatively impacts quality of life and sleep.
“We look forward to progressing this Phase Ia/Ib clinical trial. It has been a great privilege to develop this novel molecule with our world-class network of leading clinical experts in the AD space and to be supported by our Japanese partner Kaken Pharmaceutical on this path.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataAn estimated 17–26 million patients suffer from moderate-to-severe AD in the US and the European Union.
Kaken owns commercial rights to NM26-2198 in some Asian territories, including Japan, while Numab owns rights to it globally, including in the US and Europe.





