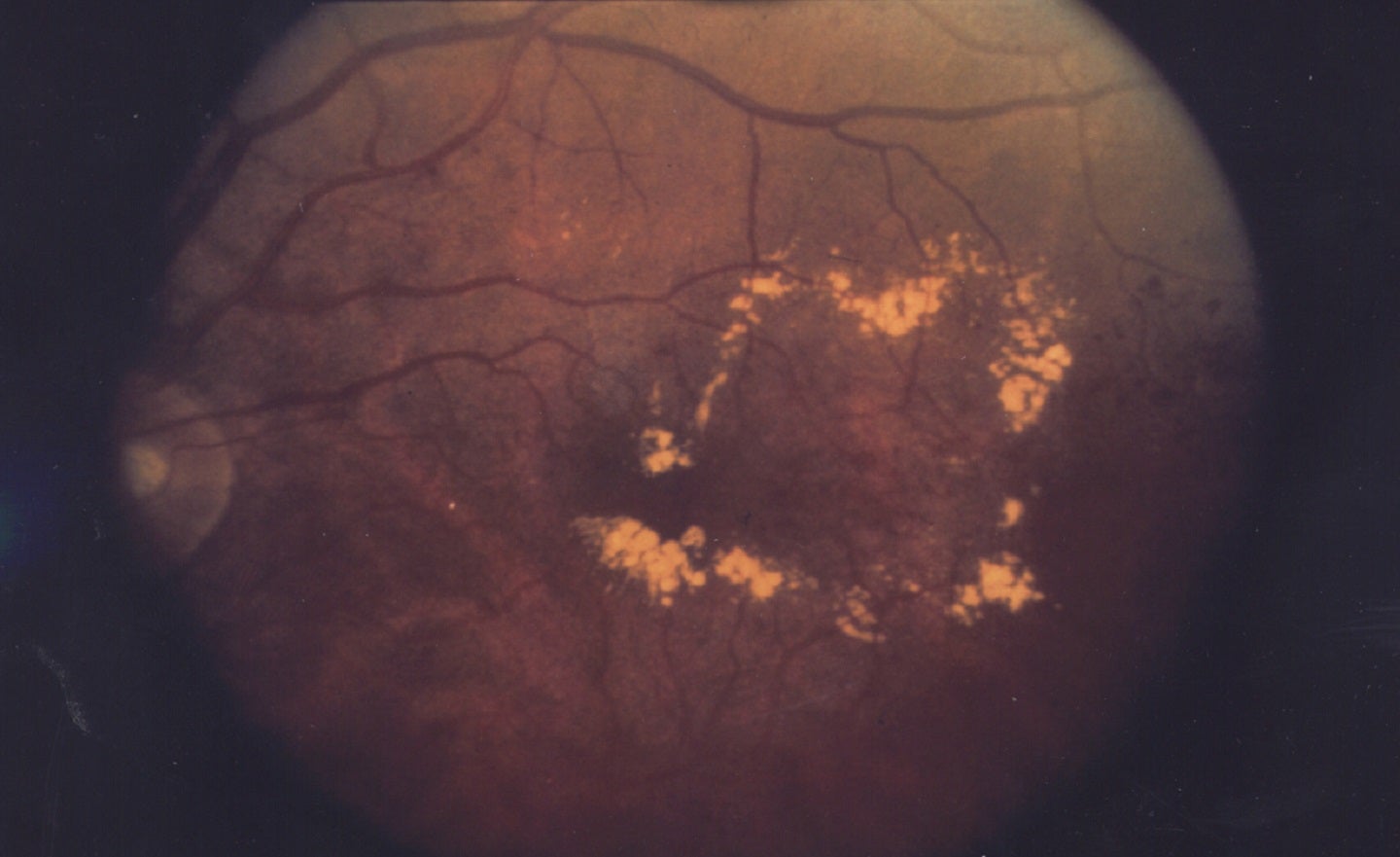
Ocugen has announced the submission of an investigational new drug (IND) application with the US Food and Drug Administration (FDA) for commencing a Phase I clinical trial of its fusion protein OCU200 to treat diabetic macular edema (DME).
The latest development fulfills the company’s commitment for filing the IND for the protein within the first quarter of this year.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The planned trial will assess the unilateral intravitreal administration of OCU200, which has a distinct mechanism of action (MOA).
It will be evaluated alone or along with an approved anti-VEGF therapy in participants with DME.
The open-label, multi-centre, dose-ranging trial has three cohorts in the dose-escalation part and one cohort in the combination therapy portion.
Ocugen chief scientific officer Dr Arun Upadhyay said: “Today’s achievement is an important step towards fulfilling our mission to bring novel therapeutics to address limitations of the current standard of care or unmet medical needs in hard-to-treat blindness diseases.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“We are encouraged by the potential for OCU200 to provide a new treatment option for the significant percentage of people living with DME, including non-responders to the current standard of care.”
Nearly 745,000 people in the US are affected with DME, a vision-threatening disease that occurs in people with diabetes and includes vision blurriness and progressive vision loss.
The company intends to pursue additional indications for the therapy for potentially treating wet age-related macular degeneration and diabetic retinopathy. Together, they affect approximately nine million Americans.
In June last year, Ocugen reported positive results from a paediatric Phase II/III clinical trial of the Covid-19 vaccine, Covaxin (BBV152), in children aged two to 18 years.





