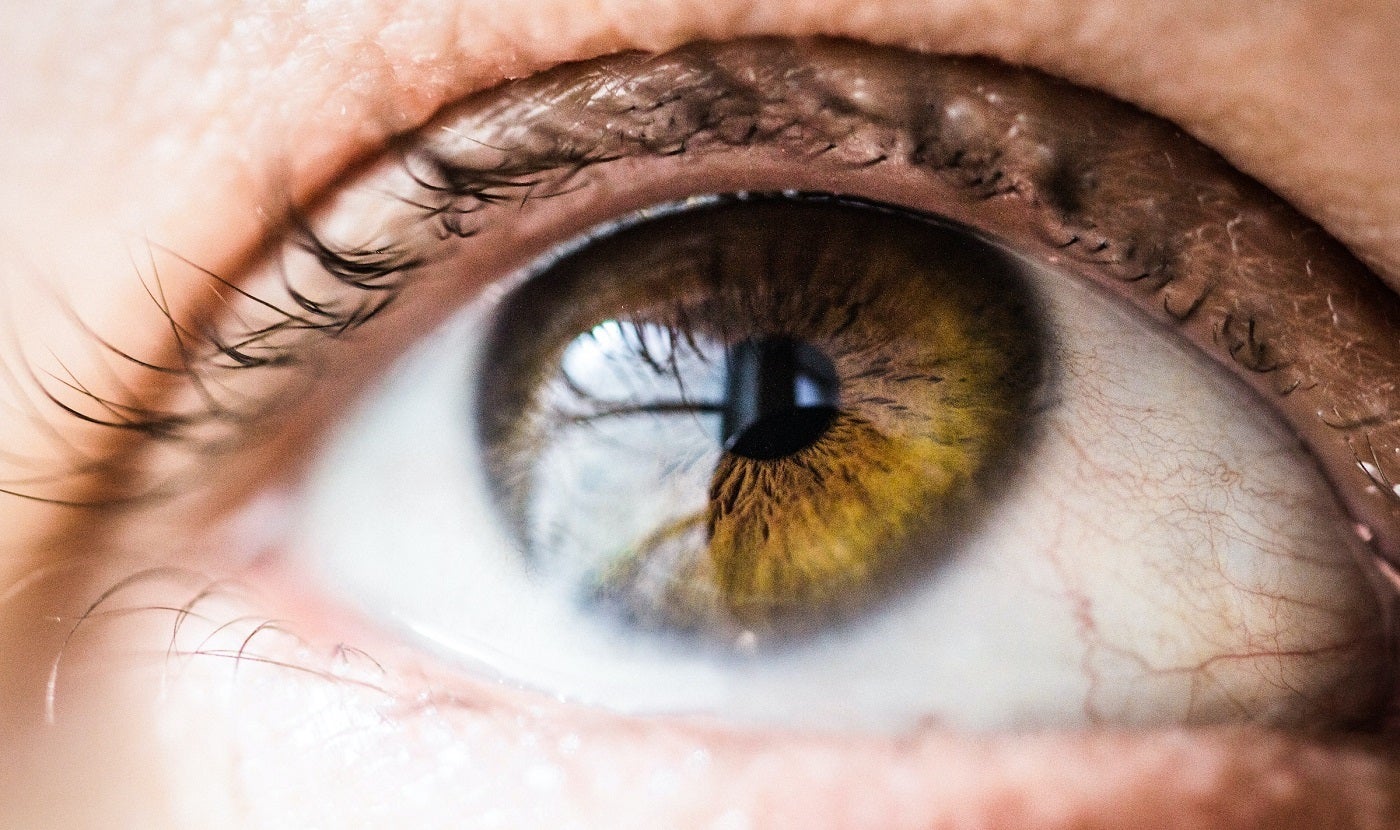
Palatin Technologies has reported data from its completed Phase II clinical trial and from the Lead In population of the ongoing Phase III MELODY-1 trial of PL9643 for the treatment of dry eye disease (DED).
The data analysis of the MELODY-1 trial was to confirm the hierarchy of the endpoints, optimal endpoints and number of patients for the final Phase III study.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
PL9643 was found to have demonstrated effectiveness across multiple clinical signs and reduced symptomatic ocular pain showing its positive affect across multiple regions of the eye.
The magnitude of the absolute difference between PL9643 and vehicle also seems to have exceeded compared to other approved products for several clinical sign and symptom endpoints.
No ocular adverse events were observed in the safety results of Phase II and initial Phase III analysis studies of PL9643 and no patients discontinued usage due to its tolerability issues.
Palatin practicing ophthalmologist and CMO Michael Raizman said: “Based on the safety, ocular tolerability, and efficacy results from the clinical studies, PL9643 may fill an important unmet need in patients with DED.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“PL9643’s novel mechanism of action potentially protects the ocular surface from the damaging effects of inflammation and helps resolve ongoing inflammation.”
The company is continuing patient enrolment for MELODY-1 Phase III study and will announce its topline data in the second half of this year.





