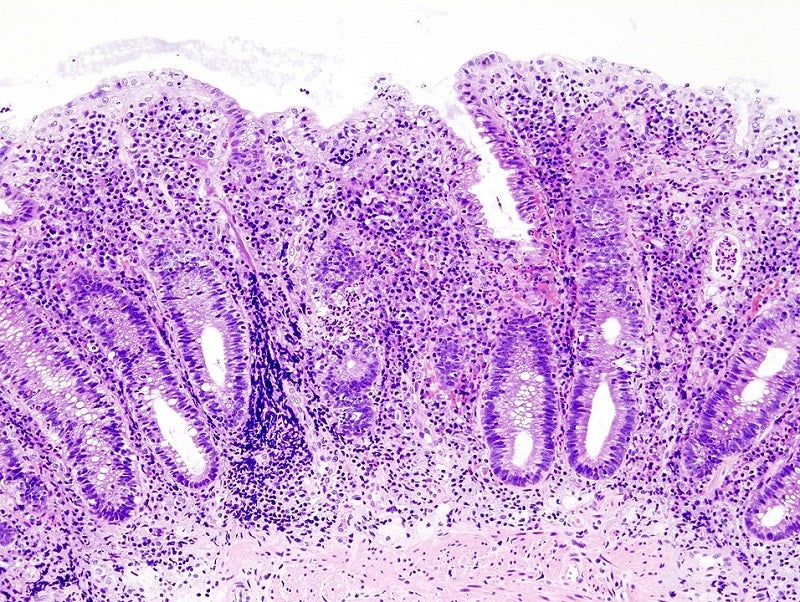
Palatin Technologies has commenced the recruitment of patients for a Phase II clinical trial of PL8177 to treat ulcerative colitis (UC).
Clinical trial sites that are taking part in the trial have been activated and the screening and recruitment of potential subjects is ongoing.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The double-blind, randomised, multi-centre, adaptive design, placebo-controlled, parallel group Phase II study has been designed to assess the tolerability, biomarkers, pharmacokinetics, safety, and efficacy of PL8177 in oral formulation in adult UC patients.
Up to 28 adult participants with active UC are planned to be enrolled from nearly 22 clinical trial locations.
In the study, all participants will be randomised and given either an oral dosing of PL8177 once a day or a placebo.
Palatin Technologies president and CEO Carl Spana said: “We are excited to advance oral PL8177, a potent, selective melanocortin-1 receptor agonist, into a Phase II clinical trial in patients with UC, an inflammatory bowel disease that affects an estimated one million people in the United States.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“The melanocortin system plays an important role in the resolution of inflammation. The oral formulation of PL8177 is targeting melanocortin-1 receptors on the luminal surface of colon epithelial cells.
“In a prior Phase I clinical study, the oral formulation successfully demonstrated sustained delivery of PL8177 to the lumen of the colon, with no systemic exposure.”
An oral potent human melanocortin receptor-1 (MC1r) agonist, PL8177 is a synthetic cyclic heptapeptide that has shown efficacy in several animal inflammatory bowel disease models.
It has been developed for resolving inflammation directly in the colon while avoiding broad immunosuppression, as well as adverse effects with selective compounds.
The oral formulation of PL8177 is designed to deliver the drug to the diseased bowel, increasing local treatment.
In the first quarter of next year, the interim assessment of PL8177-205 is expected to occur and final top line results are anticipated in the second quarter of the same year.



