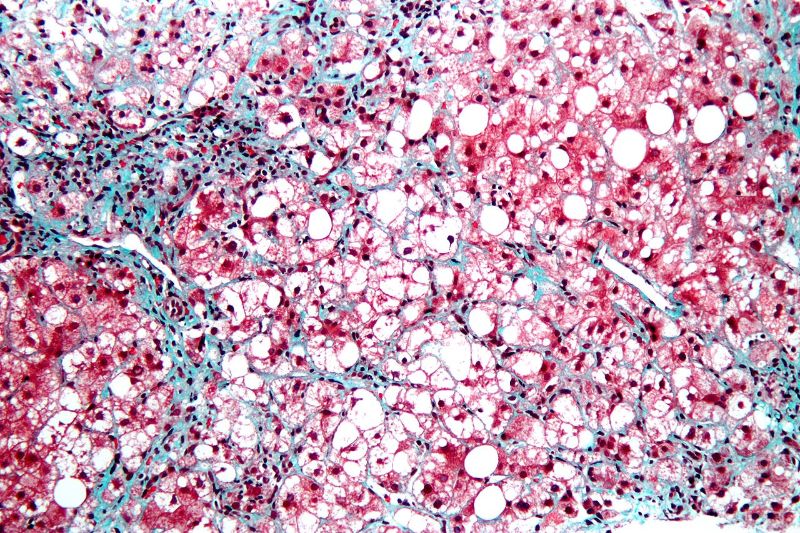
French biopharmaceutical firm Poxel has commenced a Phase Ib clinical trial for the treatment of non-alcoholic steatohepatitis (NASH) with its investigational medicine, PXL065.
PXL065 is a deuterium-stabilised R-stereoisomer of pioglitazone that inhibits the mitochondrial pyruvate carrier (MPC). It is being developed to treat non-cirrhotic patients with NASH.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The double-blind, randomised, placebo-controlled Phase Ib trial is being conducted to evaluate the safety, tolerability and pharmacokinetics (PK) of the candidate in around 30 healthy volunteers.
During the multiple ascending dose (MAD) study, participants will receive 7.5mg, 15mg and 30mg doses of PXL065 or 45mg Actos (pioglitazone) over seven days.
Results from the trial, which is expected to allow dose selection for a future study, are expected to be available in the fourth quarter of this year.
Poxel CEO Thomas Kuhn said: “We continue to believe strongly in the role of mitochondrial metabolism for the treatment of NASH and anticipate top-line data from this Phase Ib study in the fourth quarter of this year.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“In addition, PXL065 has the potential for expedited development through the 505(b)(2) regulatory pathway, and we will be meeting with the FDA early in the fourth quarter to discuss the registration programme.”
In a previous single ascending dose (SAD) Phase Ia study, three PXL065 doses were compared for their safety, tolerability and PK with 45mg Actos in 24 healthy participants.
Data revealed a favourable safety and tolerability profile for the drug candidate, without any serious adverse events. PK analysis revealed a dose-proportional increase in PXL065 plasma exposure up to 22.5mg after oral administration.
The company added that stabilisation of R-pioglitazone with deuterium was confirmed at all doses evaluated during the study.





