PTC Therapeutics is planning to hold talks with the US Food and Drug Administration (FDA) on approval after the latest Phase II Study 045 of its drug, Translarna (ataluren), failed to significantly improve dystrophin expression in patients with nonsense mutation Duchenne muscular dystrophy.
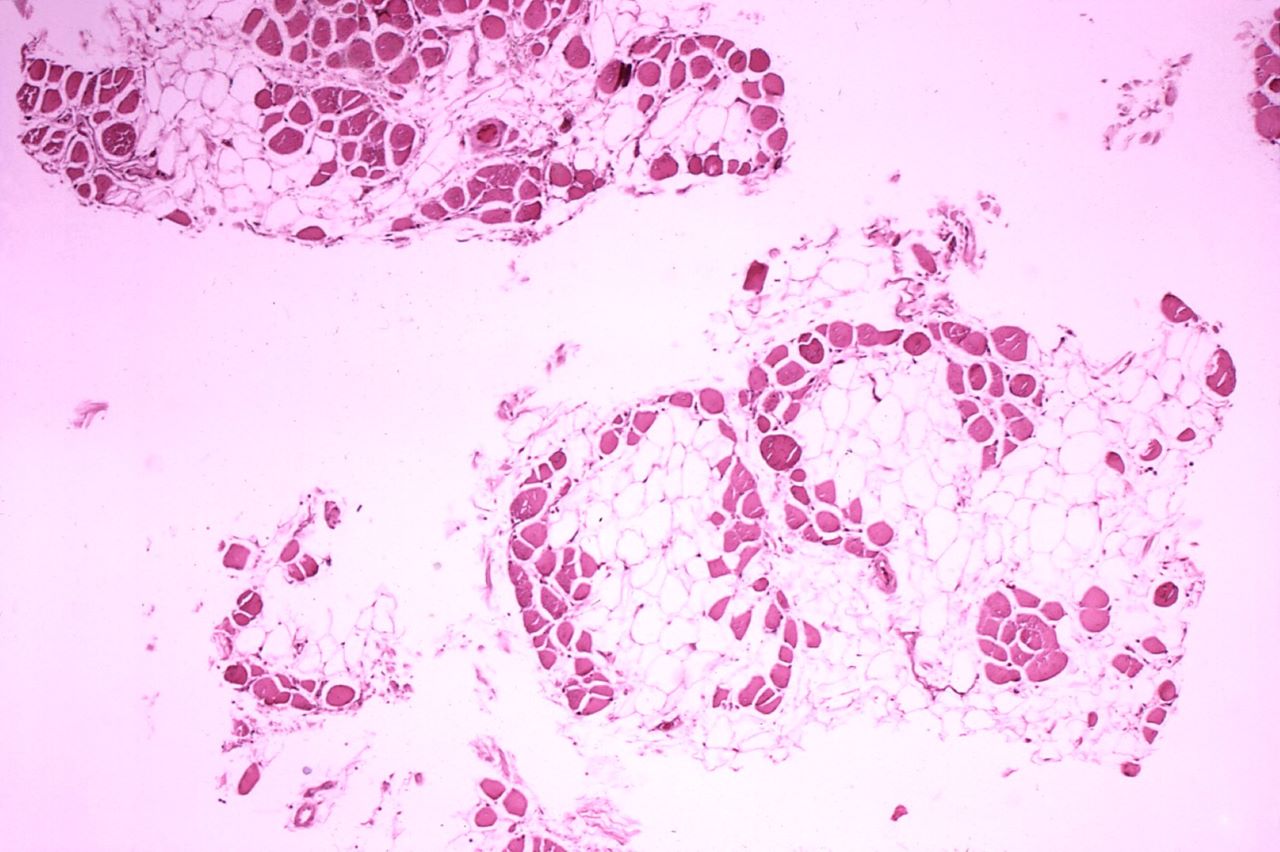
Dystrophin was measured on 20 subjects using either of the assays in the trial.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Discovered and developed by PTC, Translarna is a protein restoration therapy designed to allow the formation of a functioning protein in patients with genetic disorders caused by a nonsense mutation.
A nonsense mutation is an alteration in the genetic code that prematurely halts the synthesis of an essential protein.
A rare and fatal genetic disorder, Duchenne muscular dystrophy mainly affects men and causes progressive muscle weakness from early childhood, leading to premature death in the mid-20s due to heart and respiratory failure.
This progressive muscle disorder, caused by the lack of functional dystrophin protein, is vital for the structural stability of all muscles.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataAccording to a presentation by PTC, Study 045 trends support the benefit of Translarna. It noted that the treatment resulted in elevated dystrophin expression as assessed by both ECL and IHC assays.
Furthermore, longer treatment with the drug was linked to a greater increase in dystrophin expression.
Translarna also reduced CK levels and improved all clinical measures, the company noted.
Ataluren is an investigational new drug in the US. The company is trying to use the data to prove why the FDA should approve Translarna.
The FDA has so far rejected the drug three times but agreed to consider new results under accelerated review procedures, leading to this data.
Despite the fail, PTC plans to hold talks with the FDA based on other available trial data as well as the earlier research.
In a call with Wall Street analysts, PTC Therapeutics CEO Stuart Peltz said: “We’ll plan to discuss the dystrophin results, and the totality of the existing clinical and real-world data with the FDA to determine that there’s a potential accelerated path to approval.”
Translarna is licenced in the European Union for treating nonsense mutation Duchenne muscular dystrophy in ambulatory patients aged two years and above.





