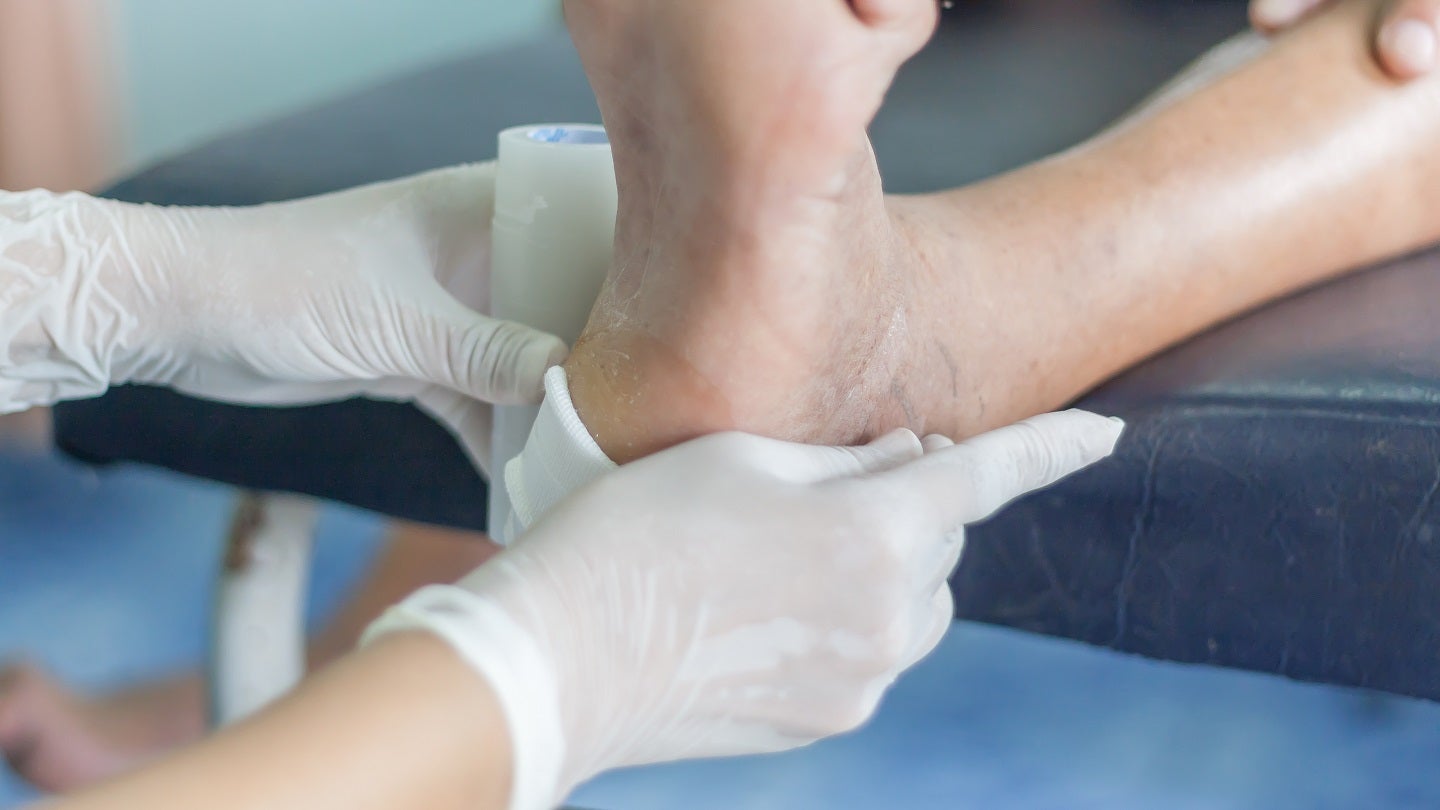
Recce Pharmaceuticals has started patient dosing in the Phase I/II proof of concept (PoC) study of RECCE 327 (R327) to treat diabetic foot infections (DFI).
Dosing is being carried out at South West Sydney Limb Preservation and Wound Research Unit at Liverpool Hospital in Australia.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The interventional, prospective study is evaluating the efficacy and safety of RECCE 327 as a potential topical anti-infective treatment for patients with mild skin and soft tissue DFI.
A total of 32 patients aged 18 years are divided into two cohorts of 16 each and will be studied for a duration of 14 days.
Patients in the first cohort will receive spray-on RECCE327 daily while the second cohort will receive the formulation for every two days.
Depending on treatment cohort allocation, a study nurse will attend the patient’s home for daily or second daily application of topical RECCE327 solution.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataParticipants will be monitored face-to-face in the High-Risk Foot Clinic at Liverpool Hospital on days seven and 14 of the trial.
At each visit, they will be assessed for treatment-related adverse effects by the nurse and are escalated to the study team.
Change in diabetic foot wound scores, as assessed using the Diabetic Foot Infection Wound Scoring scale and the wound, ischaemia and foot Infection classification is the primary outcome measure.
Other primary outcome measures include describing local and/or systemic changes and number of participants requiring rescue therapy.
Recce Pharmaceuticals CEO James Graham said: “R327 topical dosing of multiple patients in Australia’s largest DFI study is another welcomed advance to the company’s infectious disease portfolio of clinical programmes.”
RECCE 327 is an intravenous and topical therapy that targets gram-positive, and gram-negative bacteria and their superbug forms thereby treating life-threatening infections.





