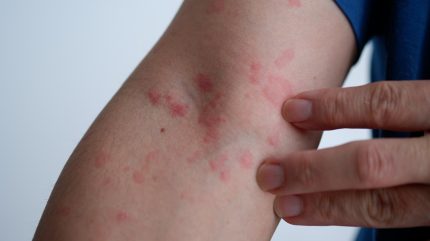
Sanofi has reported that its Phase III LIBERTY-CUPID Study C of Dupixent (dupilumab) in patients with chronic spontaneous urticaria (CSU) met primary and key secondary endpoints.
The study focused on biologic-naïve patients with uncontrolled CSU receiving background therapy with antihistamines.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Study C, part of the LIBERTY-CUPID Phase III programme, was a double-blind, randomised, placebo-controlled trial.
It assessed the efficacy and safety of Dupixent as an add-on to standard-of-care antihistamines versus antihistamines alone in 151 patients aged six years and above.
The primary endpoint was the change from baseline in itch severity at 24 weeks.
Additionally, a key secondary endpoint included the change from baseline in both itch and hives at the same timeframe.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataResults at 24 weeks showed an 8.64-point decline in itch severity following Dupixent treatment compared to a 6.10-point reduction with placebo.
For urticaria activity, there was a 15.86-point reduction with Dupixent versus an 11.21-point reduction with placebo.
Notably, 30% of patients treated with Dupixent reported no urticaria, which was a complete response, compared to 18% of those on placebo.
The safety results were found to be generally in line with the known safety profile of Dupixent in its approved indications in the dermatology field.
Treatment-emergent adverse events were observed at a rate of 53% for both Dupixent and placebo arms.
Injection site reactions, accidental overdose, and Covid-19 infection were found to be the adverse events observed with Dupixent in the trial.
This positive outcome confirms the results from Study A, the first Phase III study of Dupixent in this setting.
Earlier this year, Japan approved Dupixent for adult and adolescent CSU patients based on results from Study A.
Sanofi Development global head and chief medical officer Dietmar Berger said: “The positive pivotal data from this study reinforce the potential of Dupixent to offer a new treatment option for the many people suffering from chronic spontaneous urticaria who do not respond to standard-of-care antihistamines.
“With clinically meaningful reductions in itch and hives for patients receiving Dupixent, we look forward to sharing these data with the FDA to bring Dupixent to patients with CSU in the US as soon as possible. With Dupixent now treating one million patients across seven approved indications, these new results underscore there are still many more patients that Dupixent can potentially benefit.”
The latest development comes after the company reported data from the Phase III trials of tolebrutinib for treating multiple sclerosis.



