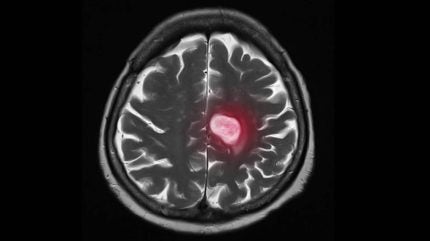
Maryland-based drug discovery company Shuttle Pharma has announced the expansion of a Phase II trial of ropidoxuridine for glioblastoma patients as the first cohort is dosed at cancer centres across the US.
Designed to be used in combination with radiation therapy, ropidoxuridine is being investigated as a treatment for glioblastoma, a form of malignant brain cancer that the National Brain Tumor Society estimates affects approximately 10,000 Americans every year with only a 6.9% chance of survival.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The Phase II randomised trial (NCT06359379) will consist of 40 patients at first, randomly sorted into two groups that will receive two different doses of ropidoxuridine, either 1,200mg a day or 960mg a day. Once the right dosage is determined, the trial will then add 14 patients at the optimal dose with the to evaluate overall survival as compared to historical controls.
The trial will target one of the most aggressive brain tumours such as IDH wild-type and methylation-negative glioblastomas, for whom radiation therapy is currently the only standard of care and will take place at the University of Virginia Cancer Center (UVA) alongside multiple other sites including the Hackensack University Medical Center, Allegheny Health Network (AHN) Cancer Institute and Miami Cancer Institute.
Shuttle Pharma CEO, Anatoly Dritschilo, said: “I am pleased with the progress being made to advance our Phase II trial of ropidoxuridine for the treatment of patients with glioblastoma with the addition of the first patient being dosed at UVA Cancer Center. This is the second trial location that has been announced to have treated patients.
“We have strategically aligned the trial with nationally recognised cancer centres across a variety of regions to treat patients with IDH wild-type, methylation-negative glioblastoma, the target of the clinical trial. I look forward to the continued advancement of the trial as we look to leverage radiation sensitizers to increase cancer cure rates, prolong patient survival and improve quality of life for patients suffering from glioblastoma.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataPreviously the company received orphan drug designation from the US Food and Drug Administration (FDA).
Elsewhere in the treatment of brain cancer, the Global Coalition for Adaptive Research (GCAR) has executed an agreement to evaluate AstraZeneca‘s AZD1390 compound in the ‘Glioblastoma Adaptive Global Innovative Learning Environment’ (GBM AGILE) trial. Meanwhile, the FDA has granted clearance for an Investigational New Drug (IND) application to Adaptin Bio, to commence a Phase I trial of its own glioblastoma treatment.





