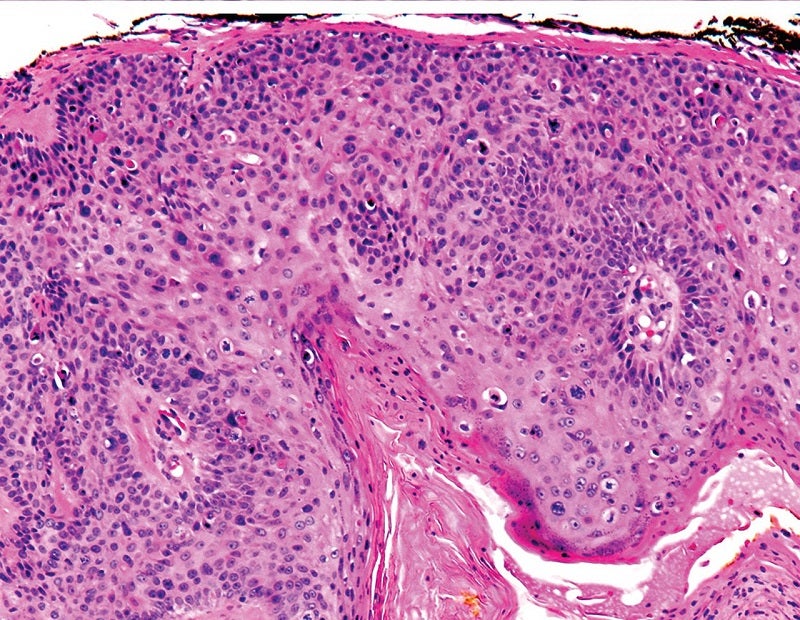
Sirnaomics has commenced a Phase IIb study to evaluate the safety and efficacy of its lead drug candidate, STP705, to treat patients with squamous cell skin cancer.
The two-part dose escalation, randomised, double-blind, placebo-controlled study will evaluate intralesional injection of the small interfering RNA therapeutic STP705 in 100 adult patients with squamous cell carcinoma in situ (isSCC).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The 30ug and 60ug dosing regimens from the Phase IIa study of the drug will be further evaluated in the run-in period of the trial, besides a third new dose level.
Sirnaomics founder, president and CEO Patrick Lu said: “In the recently concluded Phase IIa clinical trial of STP705 in isSCC, a high rate of patients achieved histological clearance in a dose dependent manner, which is the gold standard for skin cancer.
“As we initiate the Phase IIb trial, we are hopeful to learn more about the potential of this non-surgical, non-invasive treatment for common non-melanoma skin cancer, and more broadly the promise of RNAi therapeutics in oncology.”
In the second portion of the trial, the company will assess the two most efficacious dosing regimens of the drug.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe proportion of participants with histological clearance of treated isSCC lesion at the end of treatment form the primary endpoint of the trial.
Sirnaomics chief medical officer Michael Molyneaux said: “isSCC continues to be a disease with high unmet therapeutic need where surgery is still considered the only viable treatment option for many patients.
“We hope to build on the success we have seen in the Phase IIa study, where we achieved 90% histological clearance rates in the 30ug and 60ug dosing groups.”
The company expects to report initial clinical data in the second half of this year.





