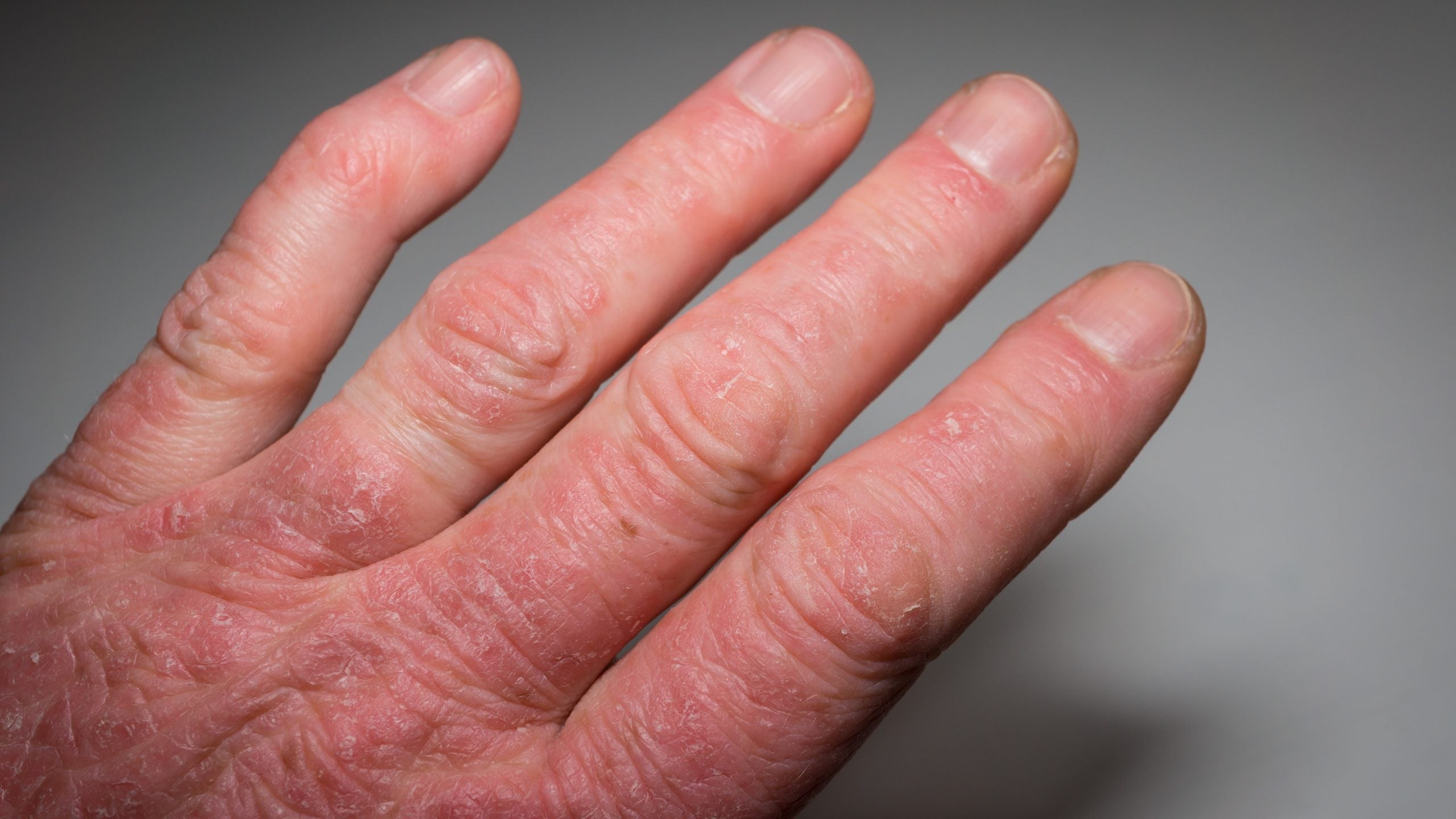
Takeda has announced it met its primary endpoint in a Phase IIb trial evaluating TAK-279 in patients with active psoriatic arthritis.
The randomised, multicentre, double-blind, placebo-controlled, multiple-dosed trial study (NCT05153148) evaluated the efficacy, safety, and tolerability of TAK-279, an investigational oral allosteric tyrosine kinase 2 (TYK2) inhibitor with next generation selectivity, in patients with active psoriatic arthritis.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
In December 2022, Takeda acquired the candidate, previously called NDI-034858 from Nimbus Therapeutics. Nimbus received a $4bn upfront payment and will also receive two milestone payments worth $1bn upon Takeda meeting annual net sales of $4bn and $5bn.
Following the positive results, Takeda’s research and development president Andy Plump said that Takeda plans to initiate Phase III studies in psoriatic arthritis and plaque psoriasis.
Plump said: “We are also advancing the development of TAK-279 in Crohn’s disease, ulcerative colitis and systemic lupus erythematosus and exploring a range of other potential indications. These opportunities are being explored in parallel with the psoriasis and psoriatic arthritis programmes.”
For plaque psoriasis, GlobalData predicts that TAK-279 will be the second approved TYK2 inhibitor for this indication and upon potential approval, will face strong competition from Bristol Myers Squibb (BMS) Sotyktu (deucravacitinib), which is a first-in-class TYK2 inhibitor approved in the US and EU.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataGlobalData is the parent company of Clinical Trials Arena.
Psoriatic arthritis trial results
The trial met its primary endpoint with a statistically significant proportion of patients, 53.3% (15mg) and 54.2% (30mg), treated with TAK-279 achieving at least an American College of Rheumatology 20 (ACR 20) response compared to 29.2% in the placebo arm at week 12 (p = 0.002).
The safety and tolerability profile of TAK-279 in the trial was consistent with that observed in the Phase IIb psoriasis study.
There were also improvements in the key secondary endpoints, including ACR 50 response, ACR 70 response, Psoriasis Area and Severity Index (PASI) 75 achievement, a reduction from baseline in the tender joint count and swollen joint, and minimal disease activity (MDA) response rates.
The most common treatment-emergent adverse events (TEAEs) were nasopharyngitis, upper respiratory tract infections, headache, and rash. Serious and grade 3 or higher TEAEs occurred infrequently and at a similar rate in the TAK-279 and placebo groups.
Compared to placebo, no opportunistic infections, major adverse cardiovascular events, or differences in mean laboratory parameters of interest were observed.
There was no clinically significant difference in adverse event rates between doses of TAK-279.





