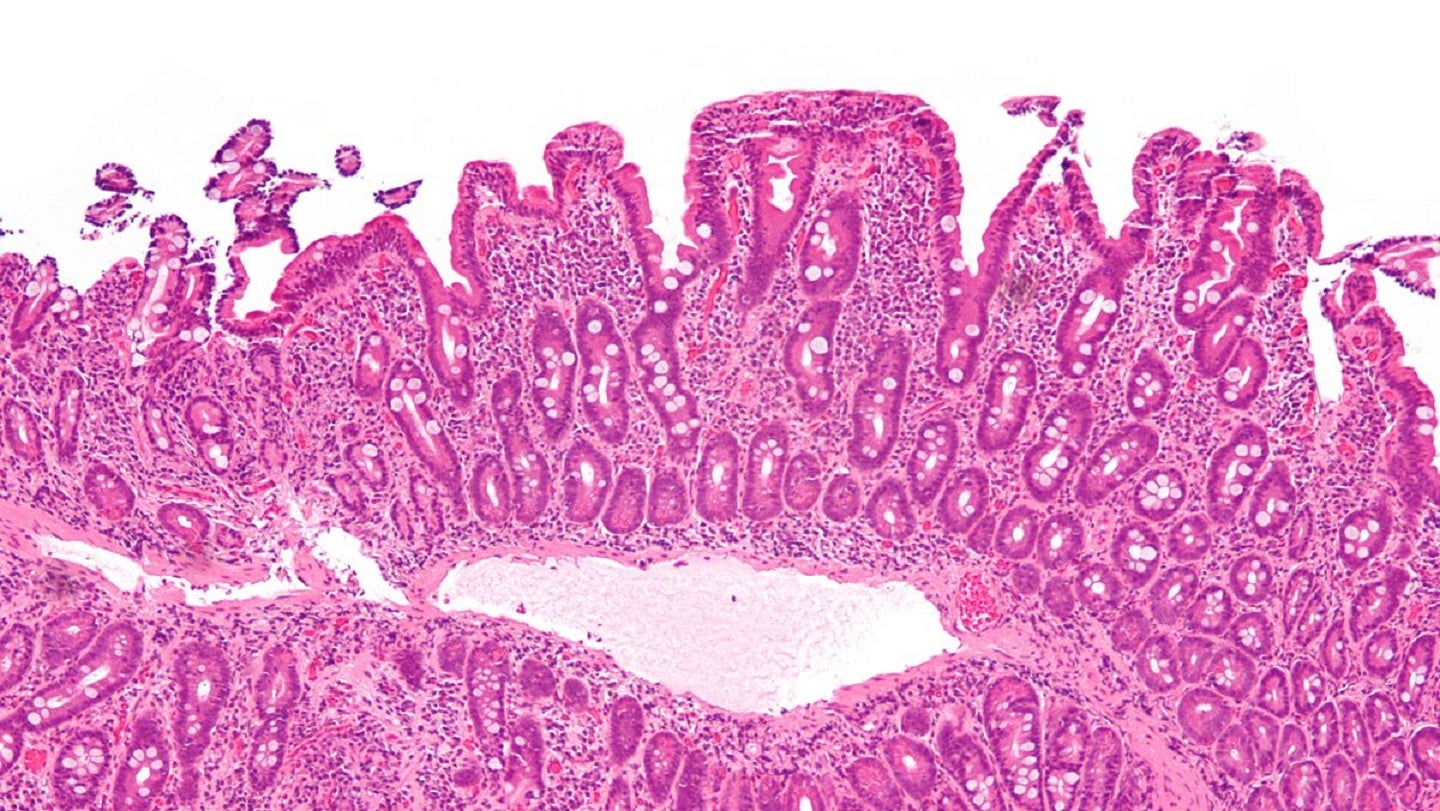
Topas Therapeutics has launched a Phase IIa clinical trial of TPM502 to treat patients with celiac disease, an autoimmune disorder.
The placebo-controlled, double-blind, randomised, multi-centre study will assess the tolerability, safety and pharmacodynamic effects of two TPM502 administrations in dose-escalating cohorts.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It will enrol 42 adults diagnosed with celiac disease.
Symptom severity and immune tolerance will be evaluated in the trial following exposure to gluten.
Topas Therapeutics chief medical officer Cristina de Min said: “The Phase IIa study will determine the capacity of TPM502 to promote immune toleration in celiac disease patients and will confirm the safety profile of TPCs.
“It will also further establish the potential of the Topas platform to generate novel therapeutics for the treatment of T-cell-mediated diseases.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“The development of TPM502 is a significant step forward in the advancement of our immune tolerance-based clinical pipeline and illustrates our mission to bring novel disease-modifying treatments to patients with limited pharmacological options.”
TPM502 utilises the company’s Topas Particle Conjugates nanotechnology comprising a proprietary biocompatible polymer and conjugated disease-relevant antigens.
It is a mixture of nanoparticles that carry gluten-specific antigenic peptides including the major gluten epitopes for HLA-DQ2.5 present in most patients with celiac disease.
The Phase IIa study has been launched based on the positive clinical data from the Phase I study of TPM203 in pemphigus vulgaris patients.
As the company is now moving to mid-stage clinical development, it is adding international pharmaceutical expertise to its executive management team.
Mireia Gómez-Angelats joined as chief business officer and Christian Schröter as chief operating officer.





