UK-based Vaccitech has announced the initiation of the Phase IIb trial across multiple sites in the Asia-Pacific region, following the positive final data readout from its HBV002 clinical trial.
The trial assesses VTP-300’s safety and immune response in chronic hepatitis B (cHBV) patients.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
VTP-300 consists of two components: chimpanzee adenovirus Oxford 1-vectored hepatitis B virus immunotherapeutic (ChAdOx1-HBV) and modified vaccinia Ankara-vectored hepatitis B virus immunotherapeutic (MVA-HBV).
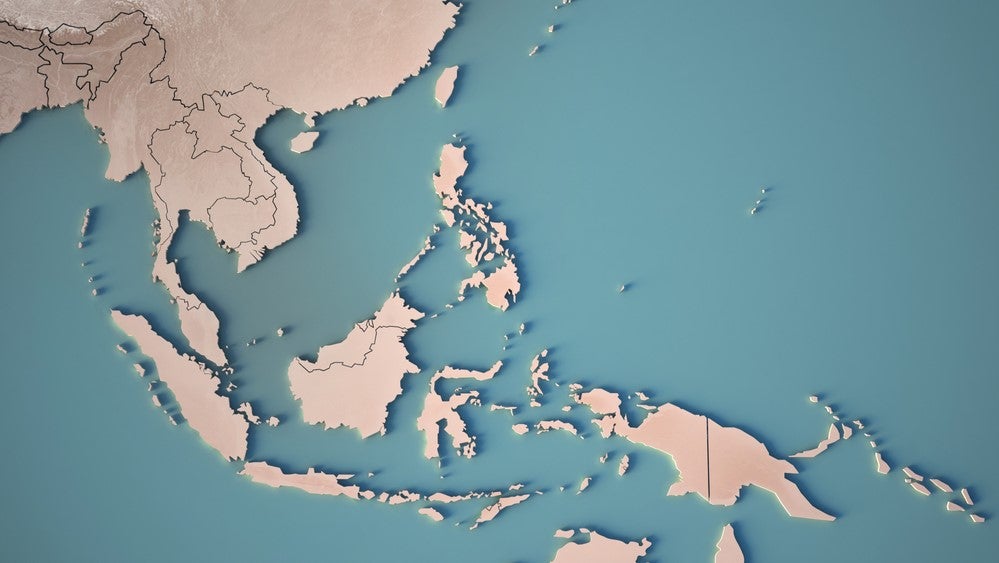
Vaccitech has initiated the Phase IIb randomised, open-label HBV003 trial (NCT05343481) to evaluate VTP-300, with additional doses of the MVA-HBV component (compared to HBV002 trial), combined with Opdivo and nucleos(t)ide analogues (NA). More than 40% of the expected 120 participants have been enrolled at multiple sites in Hong Kong, Taiwan, and Thailand, with interim data expected in the last quarter of 2023.
According to GlobalData, the Asia-Pacific region leads in the number of clinical trials for hepatitis B, with more than 250 clinical trials currently registered in the region.
GlobalData is the parent company of Clinical Trials Arena.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe HBV002 (Phase Ib/IIa randomised, open-label) clinical trial (NCT04778904) for VTP-300, with or without the addition of Opdivo (nivolumab), showed reduced Hepatitis B Surface Antigen (HBsAg) levels. The detailed data will be presented at the European Association for the Study of the Liver (EASL) Congress 2023 on 24 June in Vienna, Austria.
Mean HBsAg reductions of 0.76 log10 (at three months) were observed in 18 patients in the VTP-300 monotherapy and VTP-300/Opdivo combination therapy groups. Two patients had non-detectable HBsAg levels, signifying a decreased risk of cHBV’s deterioration.
There were no treatment-related adverse events with VTP-300 mono-therapy and combination therapies.
Vaccitech chief medical officer Meg Marshall said: “The durable reductions in HBsAg we saw in this study are exciting because they support the idea that VTP-300 could be a critical component to enhancing rates of a functional cure for people with chronic hepatitis B.”
Vaccitech has partnered with Arbutus to evaluate VTP-003 in combination with Arbutus’ AB-729 in a Phase IIa trial (ACTRN12622000317796) in virologically suppressed cHBV. The interim data from 40 patients are expected in the fourth quarter of 2023.





