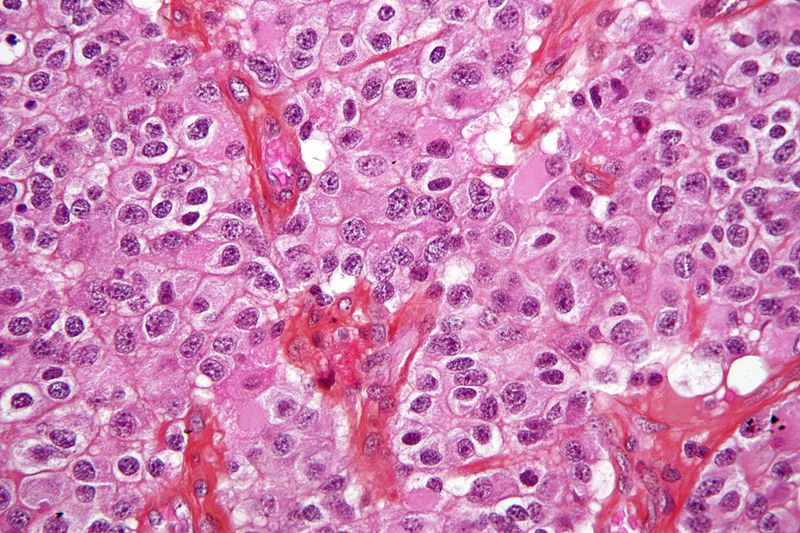
WPD Pharmaceuticals and CNS Pharmaceuticals are set to initiate a Phase I clinical trial for Berubicin, an anthracycline, in paediatric brain cancer at the Children’s Memorial Health Institute in Poland.
The companies are currently working with experts at the institute to complete documentation for the upcoming study and scientific advice meeting.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
WPD and CNS also signed a sublicense agreement, which saw WPD receive commercial rights in a limited territory to Berubicin, including research and development.
The company subsequently secured a grant of $6m from the EU/Polish National Center for research and development.
These funds will be used for the upcoming Phase I trial. CNS is also fully supporting WPD to execute these studies.
WPD Pharmaceuticals CEO Mariusz Olejniczak said: “We look forward our collaboration with CNS and Children’s Memorial in Poland to initiate what we believe to be the first investigation of a unique topoisomerase II inhibitor that appears to be able to cross the blood-brain barrier in paediatric brain tumours.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“We will continue to drive the clinical development of Berubicin in the upcoming Phase II trial in adult patients.”
Treatment of brain cancer patients with Berubicin is believed to have demonstrated positive responses that include one durable complete response in Phase I human clinical trial conducted by Reata.
Four of WPD’s ten novel drug candidates are currently in the clinical development stage. These drug candidates were researched at MD Anderson Cancer Center, Mayo Clinic, and Emory University.
The company currently has ongoing collaborations with Wake Forest University, as well as hospitals and academic centres in Poland.





