
Clinical trials data platform provider Yonalink has today announced the launch of the Israel Clinical Trials Network (ICTN), a consortium of hospitals and clinical research organisations in Israel.
The ICTN has been launched with the support of the Israel Innovation Authority and the Ministry of Health. It aims to establish Israel as an attractive prospect for clinical trial research investment, for both global pharmaceutical and medical device companies.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
“Given the digitised nature of Israel’s healthcare system, a robust innovation ecosystem and significant government investment in health tech infrastructures and biotech industries, the country is primed to be a global powerhouse for clinical trial research,” said Shomrat Shurtz, deputy VP of the growth division at Israel Innovation Authority.
She continued: “Most of the world’s largest pharmaceutical companies already have a presence in Israel, and organised cooperation among all stakeholders will ensure that clinical trials can be conducted efficiently at a national level.”
Earlier this year, Yonalink announced the results of the largest completed electronic health records (EHR) to electronic data capture (EDC) live pilot study to date, conducted in partnership with Sheba Medical Center. The study demonstrated that 93% of the requested study data was transferred and that all data was transferred with 100% accuracy.
The company hopes that its EHR-to-EDC technology can resolve the issue of interoperability in the sector, by automating patient data collection from EHRs and replacing manual data collection processes. Data is then streamed directly from the EHR to the sponsor’s EDC system, cutting out the potential for manual errors, whilst allowing real-time data oversight.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataParticipating hospitals in the ICTN include Sheba Medical Center, Rabin Medical Center, Soroka Medical Center, Assuta Hospital, Rambam Hospital, and Hadassah Medical Center.
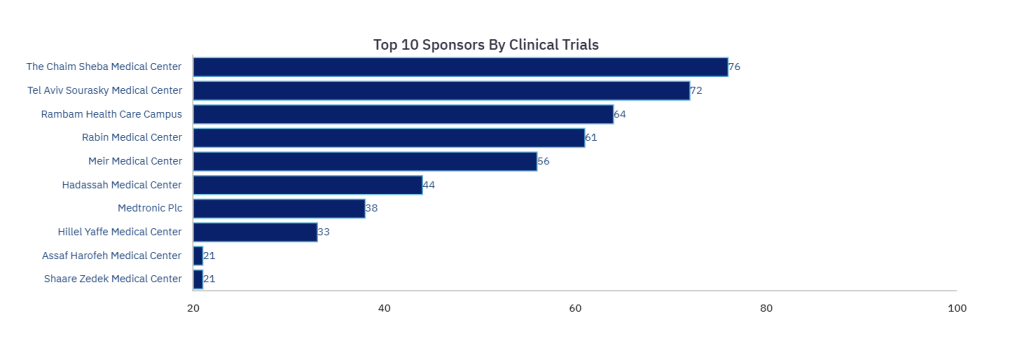
The Chaim Sheba Medical Center is a leader in the sponsorship of clinical trials for medical devices in Israel, having sponsored a total of 76 trials in its home country since 2010, according to GlobalData figures.
GlobalData is the parent company of Clinical Trials Arena.
Of the other hospitals participating in the ICTN, Rambam Hospital, Rabin Medical Center and Hadassah Medical Center are also leaders in medical device trial sponsorship across Israel, having sponsored 64, 61 and 44 respectively. This is out of a total of 1,621 medical device trials that have started in Israel since 2010.
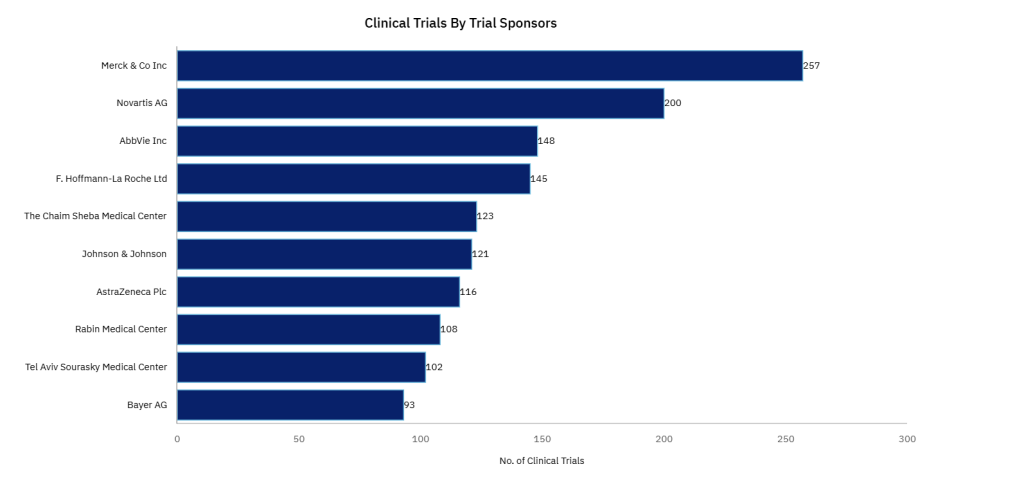
In pharma, the biggest sponsors remain private companies, but the Chaim Sheba Medical Center and Rabin Medical Center still emerged in the top ten sponsors of clinical trials in Israel by number since 2010.
GlobalData figures reveal that the Chaim Sheba Medical Center sponsored 123, whilst Rabin Medical Center sponsored 108. This is out of a total of 4,260 pharmaceutical trials that have started place in Israel since 2010.
On the ICTN Yonalink CEO and co-founder Iddo Peleg said: “Yonalink is proud to have initiated and led this collective effort to make Israel a trailblazer for clinical trial innovation and to drive investment. We see it as imperative to make life-saving drugs more accessible.
“Israel is the first country in the world to be connected at a national level for clinical trials, and our technology is powering this. Yonalink enables medical centres and pharma companies to easily stream patient data from one location to another throughout a clinical trial.”





