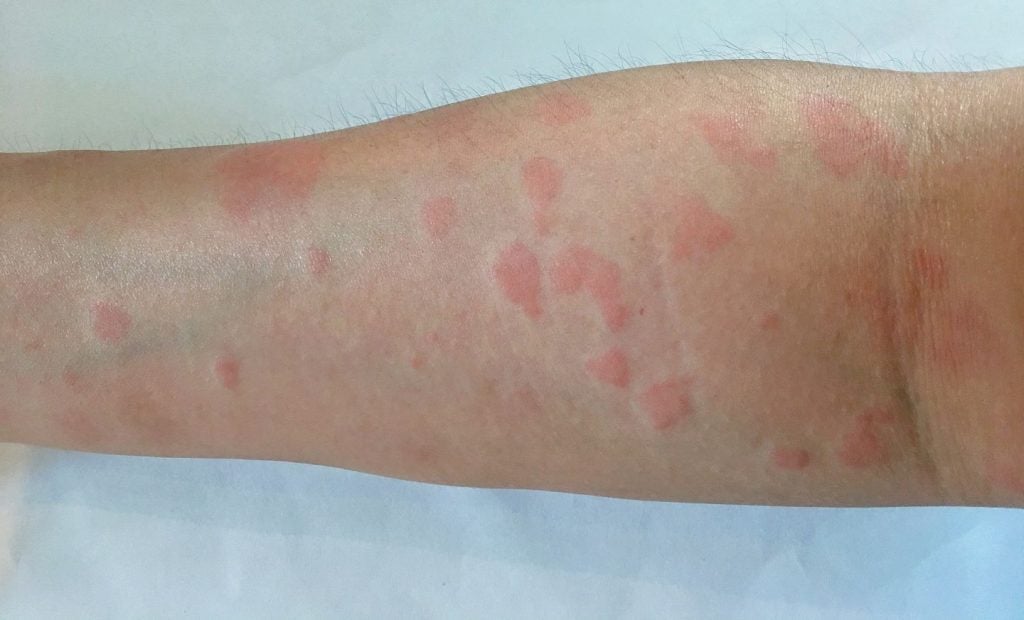
AbbVie has announced a collaboration with Lupus Therapeutics to increase clinical trial recruitment activities in its systemic lupus erythematosus (SLE) trial.
The alliance is intended for the Phase III clinical programme of AbbVie’s upadacitinib (RINVOQ) in SLE patients.
Under the collaboration, Lupus Therapeutics will offer assistance to AbbVie for the recruitment of trial subjects, as well as the activation and engagement of trial sites via the Lupus Clinical Investigators Network (LuCIN).
LuCIN comprises various academic centres in the North American region.
Based on Phase II trial data, AbbVie commenced the Phase III study of upadacitinib in SLE earlier this year.
The Phase III SELECT-SLE programme of upadacitinib in SLE patients comprises two trials.
These studies will assess the safety and effectiveness of once-a-day oral upadacitinib tablets versus a placebo, along with standard-of-care background therapy.
A long-term extension will follow these studies, the company noted.
Upadacitinib works by hindering specific signals within the inflammation-causing cells of the body.
It is currently approved for seven immune-mediated ailments in the US and is not indicated for SLE treatment.
Lupus Therapeutics executive vice-president Stacie Bell said: “The collaboration between Lupus Therapeutics and AbbVie will tap into the expertise of clinical researchers in the Lupus Clinical Investigators Network to help facilitate and improve the clinical trial process in the evaluation of upadacitinib in SLE.
“Our aim is to accelerate the development of much-needed potential treatment options for SLE, allowing people with lupus to live better lives.”