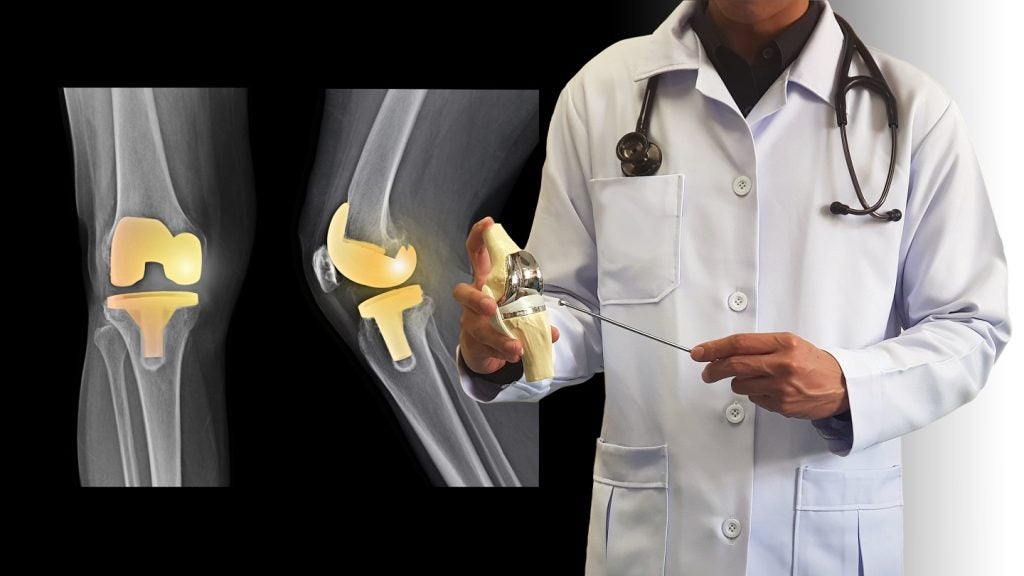
MedinCell’s partner Arthritis Innovation Corporation (AIC) has concluded enrolment of patients in the first of two Phase III trials of F14 (MedinCell codename: mdc-CWM) used for relieving localised pain after total knee replacement (TKR) surgery.
The double-blind, multicentre, randomised study intends to assess the safety and efficacy of a single dose of F14 for post-operative analgesia in patients undergoing unilateral TKR.
Arthritis Innovation CEO Dr Wayne Marshall said: “TKR is a highly invasive surgery that results in prolonged knee pain and inflammation that last for many weeks, but current single-administration, post-TKR analgesics are limited to only hours or days in their durations of efficacy.
“F14 was designed and developed to reduce that surgical pain for much longer by addressing inflammation, accelerating functional improvement, and potentially reducing opioid consumption for TKR patients.
“Completing patient enrolment in our Phase III study brings us closer to demonstrating that F14 is a first-in-class therapy that will meet that major therapeutic gap around TKR recovery.”
A total of 151 patients aged from 45 to 80 years were enrolled. They will be divided into an experimental arm that will receive F14 plus multimodal analgesia and active comparator with only multimodal analgesia.
The primary outcome measures of the study include Time-weighted Area Under The Curve (AUC) of numeric rating scale (NRS) pain scores, as assessed for two weeks.
Patients will be followed for a three-month period for conducting primary analysis. Top-line data from the study is anticipated in the first quarter of next year.
F14 is a sustained-release formulation of celecoxib, a non-steroidal anti-inflammatory drug, that is administered intraarticularly to patients at the end of TKR.
It is claimed to be the third product leveraging MedinCell's BEPO technology.