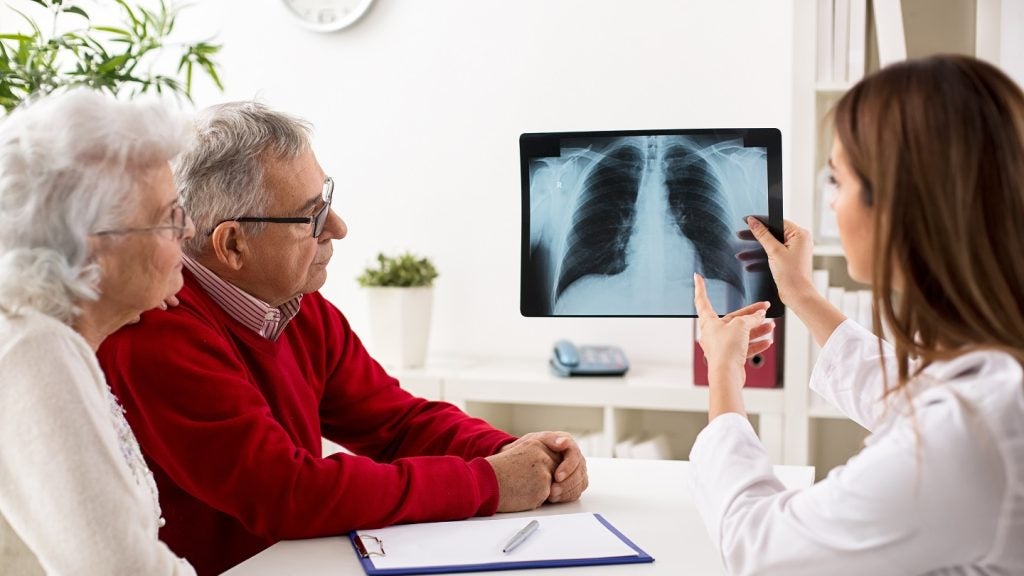
Akeso has dosed the first patient in a head-to-head Phase III trial of ivonescimab to treat locally advanced or metastatic squamous non-small cell lung cancer (sq-NSCLC).
The study will compare the safety and efficacy of the investigational PD-1/VEGF bi-specific antibody ivonescimab against tislelizumab in combination with chemotherapy as the first-line treatment of patients with sq-NSCLC.
A global, multicentre Phase III study of ivonescimab in combination with chemotherapy against pembrolizumab in combination with chemotherapy is currently underway. The study is being conducted by Akeso’s partner Summit Therapeutics.
This combined therapy trial is expected to dose the first patient in the second half of this year, as first-line treatment for metastatic sq-NSCLC (HARMONi-3).
In the US and China, PD-(L)1 inhibitor together with chemotherapy is used as the current standard of care (SOC) for lung cancer.
Akeso is currently engaged in initiating various Phase III head-to-head trials comparing ivonescimab against PD-(L)1 inhibitors in Europe, China, the US, and other developed countries.
In China, the CDE has accepted Akeso’s marketing application enabling the initiation of four pivotal registrational Phase III clinical trials, including three head-to-head trials of ivonescimab with PD-1 monoclonal antibody as the positive control drug.
Ivonescimab combines the effects of immunotherapy through a blockade of PD-1 with the anti-angiogenesis effects associated with VEGF blocking.
Akeso’s other bispecific antibody drugs include cadonilimab (PD-1/CTLA-4), PD-1/LAG-3, and TIGIT/TGF-Beta bispecific antibodies.