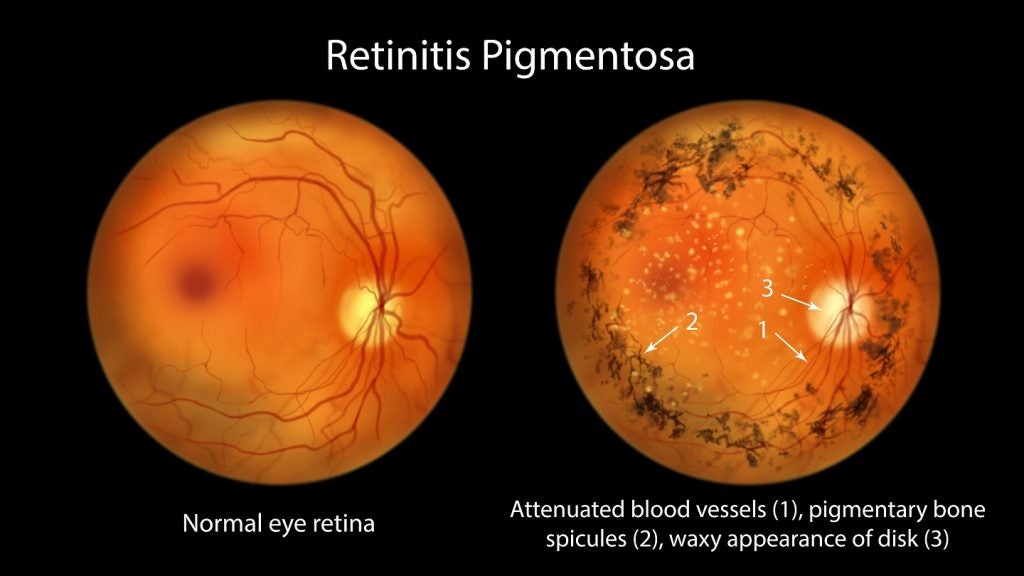
Aldeyra Therapeutics has reported positive top-line results from the Phase II clinical trial of intravitreal ADX-2191 (methotrexate injection, USP) in retinitis pigmentosa patients.
The single-centre, open-label Phase II clinical trial was conducted in eight retinitis pigmentosa patients, who have rhodopsin misfolding mutations.
Four patients received monthly injections and the remaining four received twice-monthly injections during a three-month treatment period with ADX-2191.
The findings showed that the treatment with ADX-2191 significantly improved retinal function across many different physiological and psychophysical assessments, from baseline.
Participants have also experienced an improvement in best-corrected and low‑light visual acuity.
Time taken for retinal response has statistically improved significantly, as evaluated by electroretinography, and retinal sensitivity has also significantly improved, as assessed by macular and dark-adapted perimetry.
With no safety concerns, ADX‑2191 was found to be well tolerated.
Aldeyra Therapeutics president and CEO Todd Brady said: “The improvement in retinal function relative to baseline observed in this retinitis pigmentosa clinical trial of ADX-2191 may offer hope to patients that today have no therapeutic options.
“Based on compelling proof-of-concept clinical activity that is consistent with a well-defined mechanism of action supported by preclinical evidence, we are excited to meet with regulatory authorities to discuss initiation of a potentially pivotal Phase II/III clinical trial, as we enthusiastically advance ADX-2191 to the next stage of development.”
The new intravitreal formulation of methotrexate, ADX-2191 is currently in clinical development to treat retinitis pigmentosa and proliferative vitreoretinopathy.
The US Food and Drug Administration granted orphan drug designation to the therapy to treat both indications.