Amber Implants has kickstarted a clinical trial investigating the safety and effectiveness of its implant designed to treat vertebral compression fractures (VCF).
The first-in-human study will test the company’s VCFix spinal system implant and is being conducted at the Clinic for Orthopaedics of the Mechernich Hospital in Germany.
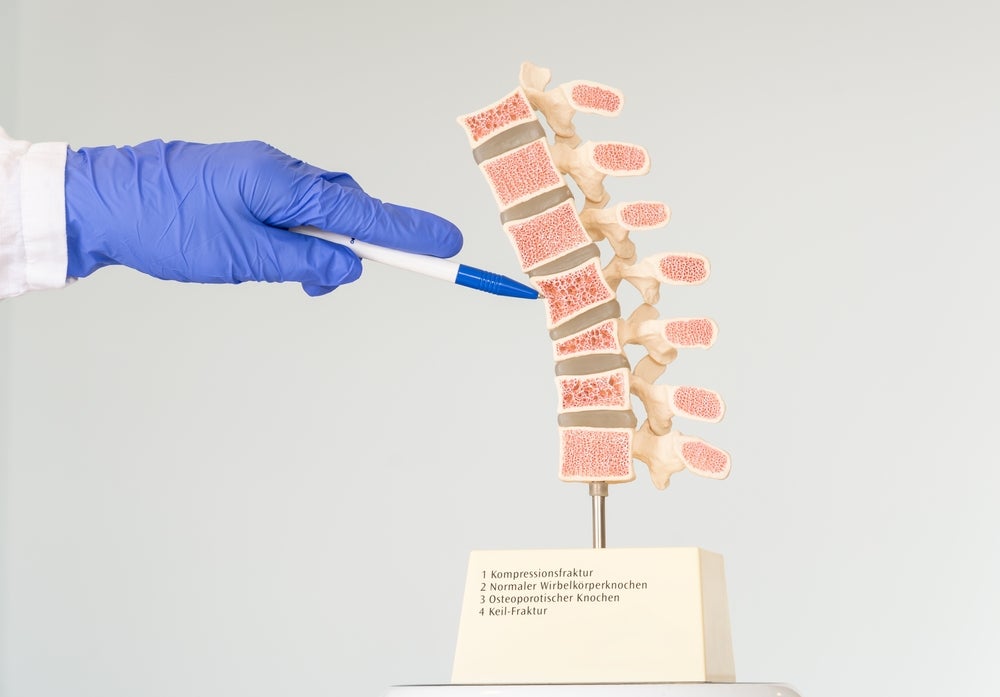
The implant for vertebral body augmentation comes with an adjustable angular opening for a personalised bone-implant interface. Amber Implants also state that, except in instances of severe and unstable fractures, the implant does not require bone cement.
Currently, injection of polymethyl methacrylate (PMMA) bone cement or multi-level posterior fixation are used to treat the 8.6 million patients who encounter different types of vertebral fractures each year.
The Hague, Netherlands-based medtech company has designed its system for both single and multi-level posterior fixation.
The vertebral compression fracture repair device market was worth $646.5m in 2022, with it estimated to rise to $1.1bn by 2033. The market is made up of vertebroplasty procedures and kyphoplasty procedures. GlobalData states the vertebroplasty market – which includes cement injection – is shrinking due to the rise of minimally invasive procedures using kyphoplasty devices.
A market model by Global reports that Medtronic dominates the global market. The medical device giant occupies nearly a 50% share globally, including 67.8% in North America. Stryker and Johnson & Johnson also possess meaningful market shares.
Dr. Banafsheh Sajadi, Co-Founder and Chief Executive Officer, Amber Implants said: “We aim to expand the clinical trial to further validate the device's versatile applications."
In August 2023, Safe Orthopaedics published data from its biomechanical study testing a new technique of a pedicle-anchored implant with standalone balloon kyphoplasty in 160 patients. The promising results were published in the Journal of Experimental Orthopaedics.