US-based biotechnology company Apogee Therapeutics has dosed the first participants ahead of schedule in a Phase I study of APG777.
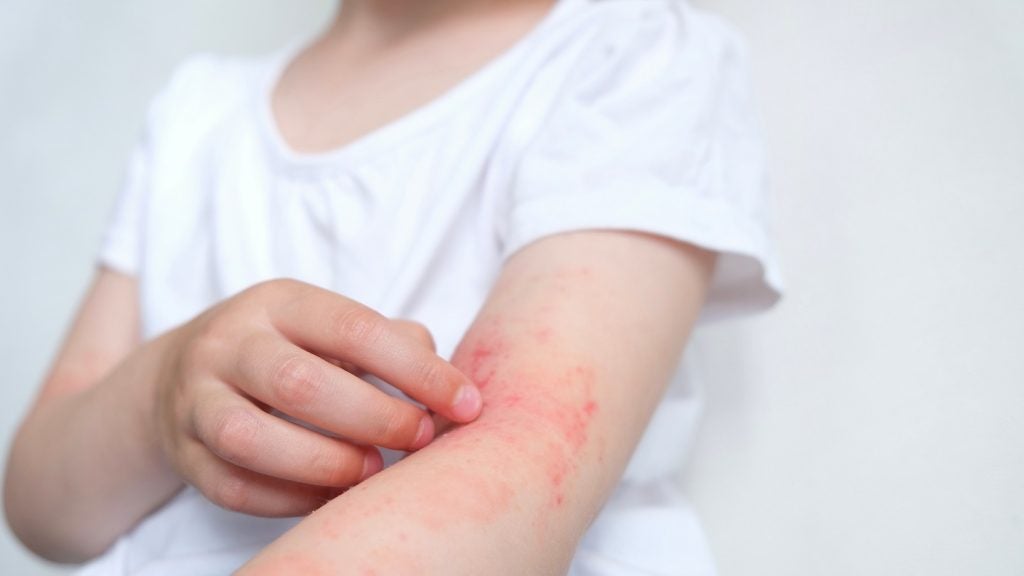
The trial aims to evaluate APG777's efficacy in adult subjects with moderate-to-severe atopic dermatitis (AD) and other inflammatory diseases.
It is being conducted as a placebo-controlled, double-blind trial that will include three single-ascending dose and two multiple-ascending dose components.
APG777 is a monoclonal antibody with an extended half-life targeting IL-13, which is a critical cytokine in inflammation and a primary driver of AD.
Apogee Therapeutics CEO Michael Henderson said: “The initiation of this Phase I study of APG777 represents an important advancement for Apogee, now a clinical-stage organisation, and for our discovery research collaboration with Paragon, a pioneer in developing best-in-class biologics for a range of diseases.
“By leveraging known targets with differentiated monoclonal antibodies, Apogee has the potential to improve the course of treatment for multiple inflammatory disorders, and APG777 is just the start of our strategy to develop a broad pipeline of potentially best-in-class product candidates.”
Around 40 healthy adult subjects will be enrolled in the trial and be given either APG777 subcutaneously or placebo.
The study’s primary endpoint is safety, while pharmacokinetics (PK) is a key secondary endpoint.
Initial safety and PK data from the trial are anticipated in the middle of next year.
Apogee chief medical officer Carl Dambkowski said: “Apogee is focused on delivering monoclonal antibody therapeutics with improved half-life and optimised potency, bioavailability and manufacturability.
“APG777 is the first realisation of these engineering efforts, with highly encouraging preclinical data that demonstrate APG777 has similar potency to current therapies, but with significantly longer half-life that could enable less frequent dosing.
“A new option providing dosing every two or three months could be a transformative change in the standard of care for moderate-to-severe AD patients.”