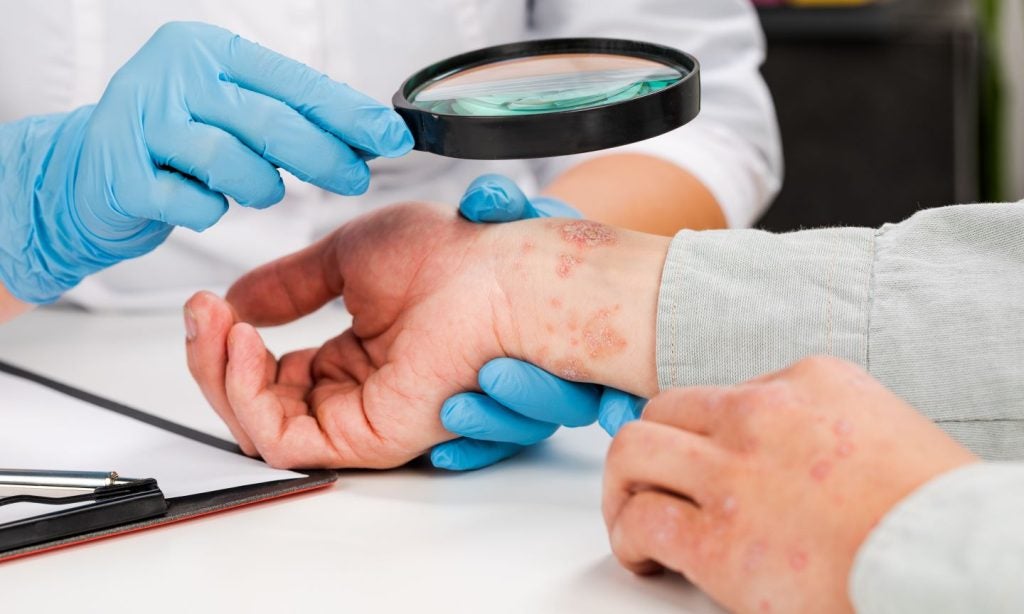
Artax Biopharma has obtained clinical trial authorisation (CTA) from the UK Medicines and Healthcare Products Regulatory Agency (MHRA) to assess AX-158 in a Phase IIa study in psoriasis patients.
With the approval, the company intends to commence subject enrolment by the end of this year at study centres in the UK.
The double-blind, randomised, placebo-controlled, proof-of-concept trial is designed to assess the tolerability and safety of AX-158 versus placebo in patients with mild to moderate plaque psoriasis.
Subjects will be randomised into a 2:1 ratio to receive AX-158 or a placebo.
The trial will enrol 30 participants who will receive the treatment for 28 days and a subsequent dosing for another additional 30 days.
An investigational oral, small molecule immunomodulator, AX-158 can adjust T cell responses vital in the healthy functioning of the immune system.
It can also regulate various cytokines that cause diseases without needing immunosuppression.
Artax Biopharma CEO Joseph Lobacki said: “We are proud to announce the clinical progress of AX-158 and the potential that it may provide a novel treatment to patients managing psoriasis.
“Additionally, we are excited by the promise of AX-158 as a new standard of care in treating a broad range of autoimmune diseases.
“In particular, we believe that AX-158 will offer the opportunity to provide a convenient once daily oral therapy that will treat disease while not impairing the ability of the patient's immune system to function properly.”