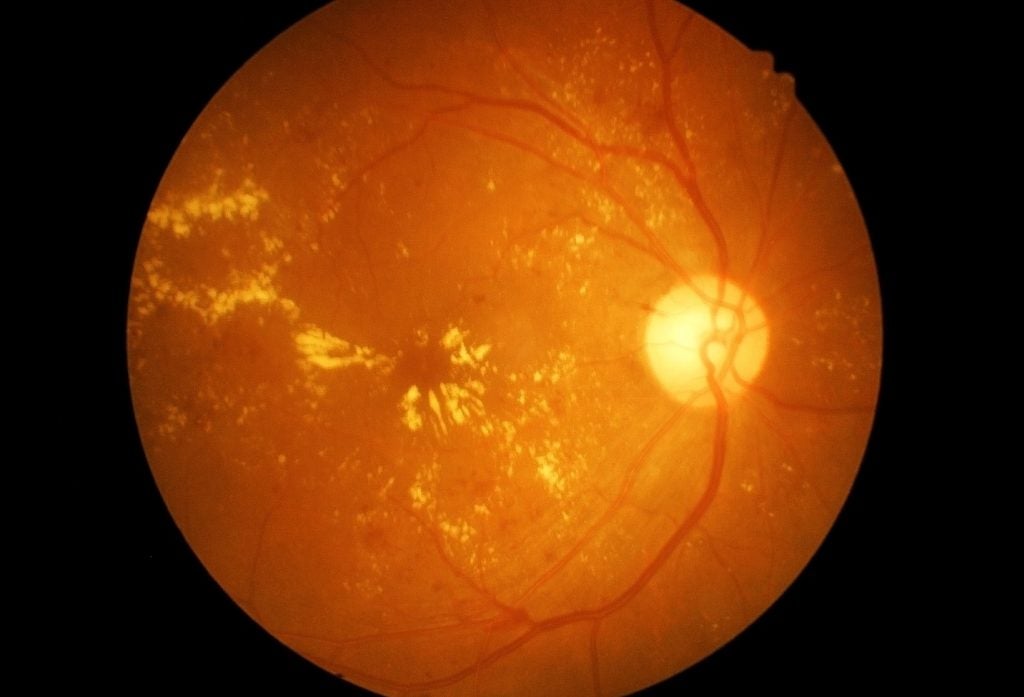
Aviceda Therapeutics has dosed the first patient in the GLYCO Phase II clinical trial in the US, aimed at evaluating AVD-104 for diabetic macular oedema (DMO; DME in the US) treatment.
AVD-104 is an engineered glycan (sialic acid) nanoparticle designed to reduce inflammation. This is done by targeting the self-pattern recognition receptors on retinal neutrophils, microglia, and macrophages that are overly activated and repolarising them to their resolution state.
The GLYCO Phase II trial will assess AVD-104’s safety and treatment effects when administered intravitreally in patients with DMO.
As part of the multi-centre, open-label safety and tolerability trial, 30 patients are planned to be enrolled to test both low and high doses of the therapy, with a three-month follow-up period.
The trial’s primary endpoint is to determine the incidences and severity of ocular and systemic adverse events.
Secondary endpoints will focus on standard treatment efficacy evaluations, including macular thickness and vision.
Aviceda co-founder and CEO Mohamed Genead said: “We are excited to evaluate the ability of AVD-104’s unique mechanism of action to provide DME patients with a safe treatment that may improve outcomes in eyes with DME driven by inflammatory and VEGF-mediated mechanisms.”
The company anticipates topline results from the GLYCO Phase II trial in the second quarter of 2024.
Aviceda chief medical officer and senior vice-president David Callanan said: “AVD-104 down-regulates neutrophils and has been shown to reduce the release of inflammatory cytokines.
“There is still a need for improvement in the treatment of DME and we believe it may offer therapeutic advantages in the management of DME, and potentially in diabetic ischemia.”
Aviceda also has a pipeline of products that are under development in ophthalmology and several other therapeutic areas, including immunology, oncology, neurology, and fibrosis.