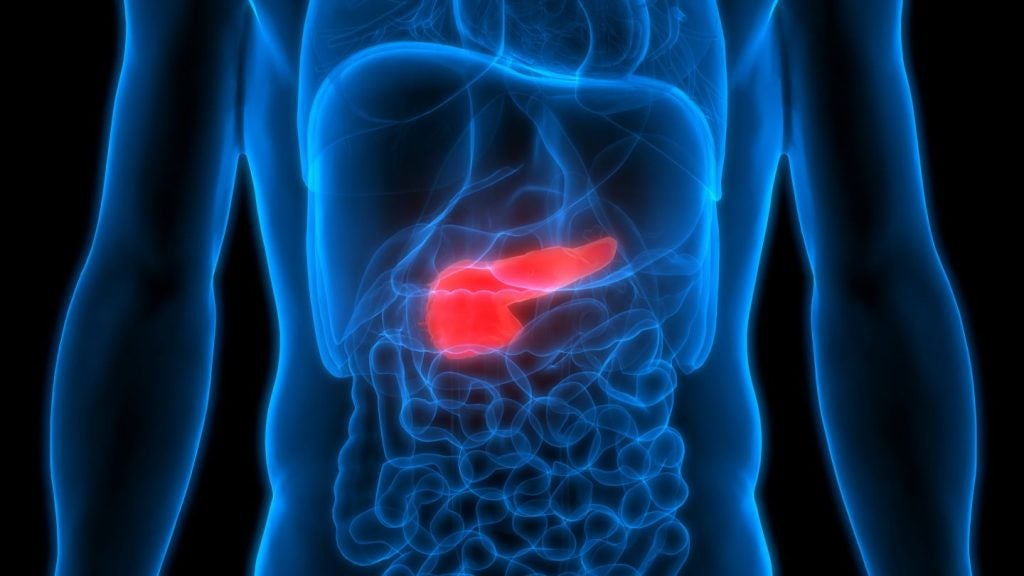
BioLineRx has dosed the first subject in the Phase II CheMo4METPANC clinical trial evaluating the CXCR4 inhibitor motixafortide for the treatment of first-line pancreatic cancer (PDAC).
The trial aims to assess the combination of motixafortide plus PD-1 inhibitor cemiplimab and standard chemotherapies -- gemcitabine and nab-paclitaxel.
The single-arm, randomised, investigator-initiated multicentre trial is sponsored by Columbia University and supported by BioLineRx and Regeneron.
It will randomise 108 patients to receive either the combination therapy or the standard chemotherapies alone.
Progression-free survival (PFS) is the trial’s primary endpoint while secondary objectives comprise response rate, safety, disease control rate, overall survival and duration of clinical benefit.
The trial marks a significant step in pancreatic cancer research as it is the first large, multi-centre, randomised study to assess motixafortide with a PD-1 inhibitor and standard chemotherapies in PDAC.
Results from the pilot phase of the study have shown promising outcomes, with 64% of subjects experiencing a partial response and a disease control rate of 91%.
A lead therapeutic candidate of the company, motixafortide has received approval from the US Food and Drug Administration (FDA) in September last year.
It is indicated for use along with filgrastim for mobilisation of hematopoietic stem cells for collection and ensuing autologous transplantation in multiple myeloma patients.
The drug is also under investigation as a single agent along with natalizumab for CD34+ hematopoietic stem cell mobilisation in gene therapies for sickle cell disease (SCD) in a Phase I trial.
BioLineRx CEO Philip Serlin said: “Pancreatic ductal adenocarcinoma (PDAC) has had limited responses to traditional immunotherapy, resulting in a poor prognosis for patients and an urgent need for new treatment approaches.
“We are encouraged by our early pilot data and look forward to continuing to advance the expanded, randomised Phase II CheMo4METPANC trial for patients living with this cancer.”