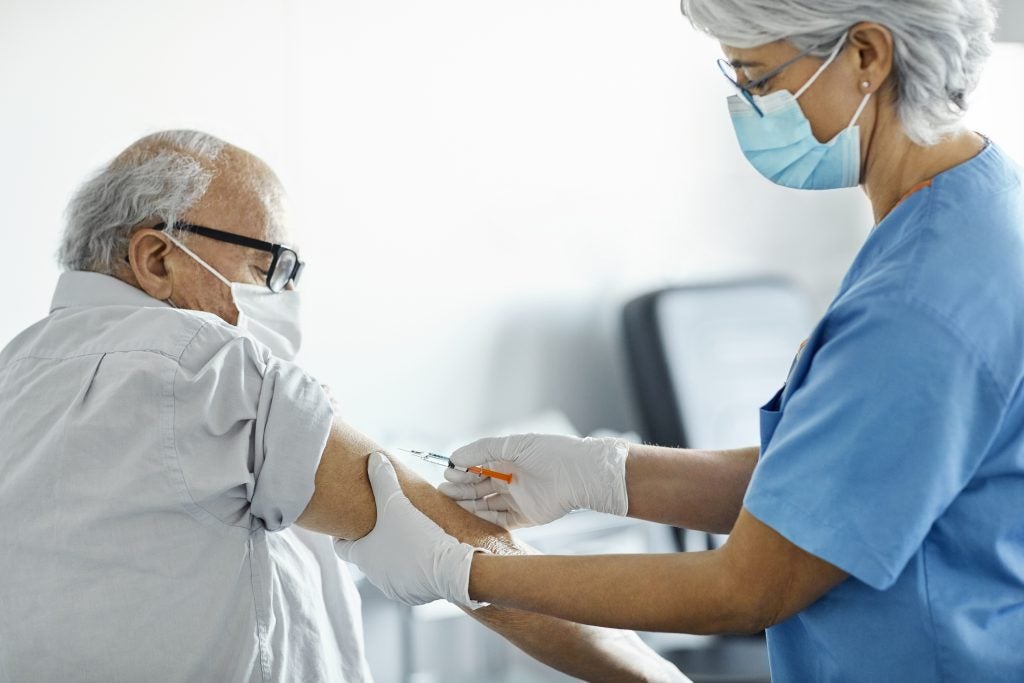
Older adults in the US might soon be able to receive protection ahead of the respiratory syncytial virus (RSV) season in autumn after advisers to the Centers for Disease Control and Prevention (CDC) recommended both GlaxoSmithKline (GSK) and Pfizer’s respective vaccines.
Across two separate votes, the CDC's Advisory Committee on Immunization Practices (ACIP) recommended adults aged 60 and above can receive the RSV vaccines.
The recommendation came with a caveat that the vaccines be used based on “shared clinical decision-making”, wherein individuals can consult healthcare providers to determine if the vaccine is right for them. The last step now lies with the CDC, which will give its final approval before GSK’s Arexvy and Pfizer’s Abrysvo are made available.
In early May, the US Food and Drug Administration (FDA) approved Arexvy for adults ages 60 and above, crowning GSK the winner in the race for the first RSV vaccine, edging out Pfizer who gained approval for its own vaccine a few weeks later. GSK and Pfizer both presented data from Phase III trials evaluating efficacy into a second RSV season.
At the ACIP meeting, GSK publicly unveiled its data from the Phase III trial (NCT04886596), demonstrating that a single dose of its vaccine exhibited an 82.6% efficacy in preventing lower respiratory tract disease (LRTD) due to RSV in the first season, which dropped to 77.3% during the second season. These results were an updated view of the data first published in February.
For severe LRTD caused due to RSV, vaccine efficacy was 94.1% during the first season and 84.6% during the second. In the statement announcing the results, GSK added that a similar pattern of efficacy was observed in adults with underlying comorbidities and advancing age.
Pfizer also presented data from its trial (NCT05035212) showing that, between the first season and the middle of the second season, Abrysvo’s efficacy in preventing LRTD dropped from 88.9% to 78.6%. For the severe disease form, efficacy fell from 65% to 49.9%.
As per Reuters, ACIP committee member Dr Helen Keipp Talbot said concerns remain about the vaccine’s effectiveness in high-risk patients: “Those who are at high risk for disease and for high risk for hospitalisations and death were actually not included in the trials. The patient population that participated in the study were younger and healthier and had fewer comorbid conditions, were not immunocompromised and were not living in nursing homes.”