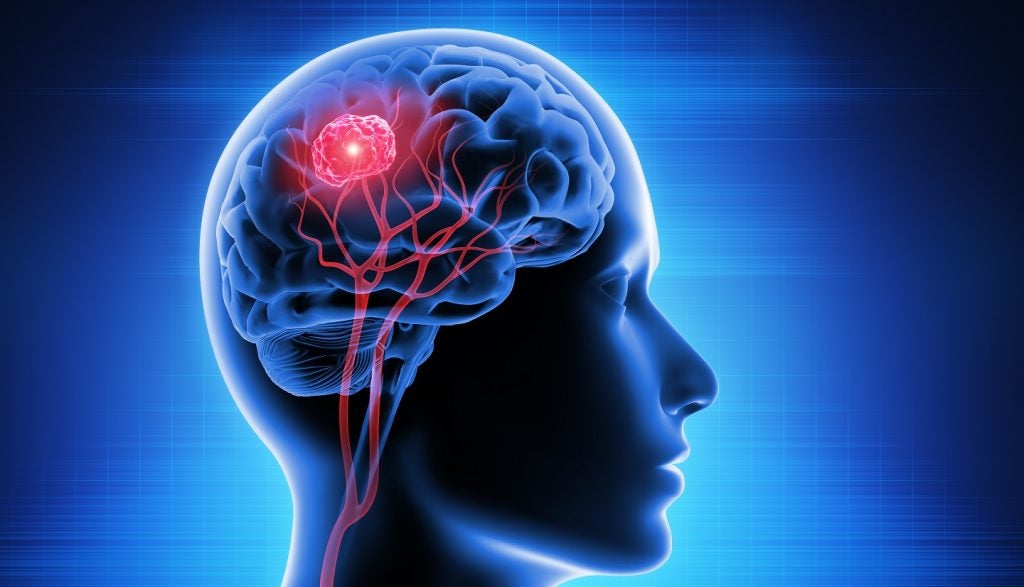
Chimeric Therapeutics has launched a new Phase IB clinical trial of CHM 1101 (CLXT CAR T) cell therapy to treat patients with recurrent or progressive glioblastoma multiforme (GBM).
The study will evaluate the safety and efficacy of CHM 1101 in patients with the deadliest primary brain cancer.
It intends to enrol patients at the Sarah Cannon Research Institute (SCRI) at St David’s South Austin Medical Center in Austin, Texas, US.
The two-part Phase IB study will determine a recommended Phase II dose and includes Part A and Part B.
Part A of the study will enrol three to six patients and test for the highest dose at the City of Hope Cancer Centre, and Part B will enrol 12 to 26 additional patients.
Chimeric will also evaluate the safety and activity from the CHM 1101 clinical programme late this year.
Chimeric Therapeutics CEO and managing director Jennifer Chow said: “This multi-centre trial will enable us to more rapidly advance the development of CHM 1101 with recruitment across multiple clinical trial sites and also prepare us to accelerate the next phase of development if supported by the clinical results.”
Upon completion of the Part B dose examination arm, Chimeric plans to design and initiate a registration trial along with global regulatory feedback.
In the initial two dose cohorts carried out at the City of Hope, Phase IA investigator-initiated trial of CHM 1101 demonstrated safety with ~70% disease stability.
Chimeric Therapeutics chief medical officer Jason Litten said: “GBM continues to represent an important unmet medical need and the early clinical results from the City of Hope trial provide support that CHM 1101 may improve outcomes for GBM patients.”